Abstract
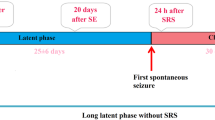
There is growing experimental evidence that tracing the elements involved in brain hyperexcitability, excitotoxicity, and/or subsequent neurodegeneration could be a valuable source of data on the molecular mechanisms triggering or promoting further development of epilepsy. The most frequently used experimental model of the temporal lobe epilepsy observed in clinical practice is the one based on pilocarpine-induced seizures. In the frame of this study, the elemental anomalies occurring for the rat hippocampal tissue in acute and silent periods after injection of pilocarpine in rats were compared. X-ray fluorescence microscopy was applied for the topographic and quantitative elemental analysis. The differences in the levels of elements such as P, S, K, Ca, Fe, Cu, and Zn between the rats 3 days (SE72) and 6 h (SE6) after pilocarpine injection as well as naive controls were examined. Comparison of SE72 and control groups showed, for specific areas of the hippocampal formation, lower levels of P, K, Cu, and Zn, and an increase in Ca accumulation. These results as well as further analysis of the differences between the SE72 and SE6 groups confirmed that seizure-induced excitotoxicity as well as mossy fiber sprouting are the mechanisms involved in the neurodegenerative processes which may finally lead to spontaneous seizures in the chronic period of the pilocarpine model. Moreover, in the light of the results obtained, Cu seems to play a very important role in the pathogenesis of epilepsy in this animal model. For all areas analyzed, the levels of this element recorded in the latent period were not only lower than those for controls but were even lower than the levels found in the acute period. The decreased hippocampal accumulation of Cu in the phase of behavior and EEG stabilization, a possible inhibitory effect of this element on excitatory amino acid receptors, and enhanced seizure susceptibility in Menkes disease (an inherited Cu transport disorder leading to Cu deficiency in the brain) suggest a neuroprotective role rather than neurodegenerative and proconvulsive roles of Cu in pilocarpine-induced epilepsy.





Similar content being viewed by others
References
Loscher W (2002) Animal models of epilepsy for the development of antiepileptogenic and disease-modifying drugs. A comparison of the pharmacology of kindling and post-status epilepticus models of temporal lobe epilepsy. Epilepsy Res 50:105–123
Sharma AK, Reams RY, Jordan WH, Miller MA, Thacker HL, Snyder PW (2007) Mesial temporal lobe epilepsy: pathogenesis, induced rodent models and lesions. Toxicol Pathol 35:984–999
Loscher W (1997) Animal models of intractable epilepsy. Prog Neurobiol 53:239–258
Scorza FA, Arida RM, Naffah-Mazzacoratti Mda G, Scerni DA, Calderazzo L, Cavalheiro EA (2009) The pilocarpine model of epilepsy: what have we learned? An Acad Bras Cienc 81:345–365
Chwiej J, Winiarski W, Ciarach M, Janeczko K, Lankosz M, Janeczko K, Rickers K, Setkowicz Z (2008) The role of trace elements in the pathogenesis and progress of pilocarpine-induced epileptic seizures. J Biol Inorg Chem 13:1267–1274
Chwiej J, Janeczko K, Marciszko M, Czyzycki M, Rickers K, Setkowicz Z (2010) Neuroprotective action of FK-506 (tacrolimus) after seizures induced with pilocarpine: quantitative and topographic elemental analysis of brain tissue. J Biol Inorg Chem 15:283–289
Chwiej J, Sarapata A, Janeczko K, Stegowski Z, Appel K, Setkowicz Z (2011) X-ray fluorescence analysis of long-term changes in the level and distribution of trace elements in the rat brain following mechanical injury. J Biol Inorg Chem 16:275–283
Paxinos G, Watson C (1989) The rat brain in stereotaxic coordinates. Academic, Chatswood
Solé VA, Papillon E, Cotte M, Walter P, Susini J (2007) A multiplatform code for the analysis of energy-dispersive X-ray fluorescence spectra. Spectrochim Acta B 62:63–68
Hill T, Lewicki P (2006) Nonparametric statistics. Statistics, methods and applications. A comprehensive reference for science, industry and data mining. StatSoft, Tulsa
Hauser WA, Annegers JF, Rocca WA (1996) Descriptive epidemiology of epilepsy: contributions of population-based studies from Rochester, Minnesota. Mayo Clin Proc 71:576–786
Wieser HG (2004) Mesial temporal lobe epilepsy with hippocampal sclerosis. Epilepsia 45:695–714
Dalby NO, Mody I (2001) The process of epileptogenesis: a pathophysiological approach. Curr Opin Neurol 14:187–192
Pitkanen A, Sutula TP (2002) Is epilepsy a progressive disorder? Prospects for new therapeutic approaches in temporal-lobe epilepsy. Lancet Neurol 1:173–181
Arundine M, Tymianski M (2003) Molecular mechanisms of calcium-dependent neurodegeneration in excitotoxicity. Cell Calcium 34:325–337
Fujikawa DG (2005) Prolonged seizures and cellular injury: understanding the connection. Epilepsy Behav 7:3–11
Scorza FA, Arida RM, Naffah-Mazzacoratti Mda G, Scerni DA, Calderazzo L, Cavalheiro EA (2009) The pilocarpine model of epilepsy: what have we learned? An Acad Bras Cienc 81:345–365
Fellin T, Pascual O, Haydon PG (2006) Astrocytes coordinate synaptic networks: balanced excitation and inhibition. Physiology (Bethesda) 21:208–215
Badawy RA, Harvey AS, Macdonell RA (2009) Cortical hyperexcitability and epileptogenesis: understanding the mechanisms of epilepsy—part 2. J Clin Neurosci 16:485–500
Chang BS, Lowenstein DH (2003) Epilepsy. N Engl J Med 349:1257–1266
McNamara JO, Huang YZ, Leonard AS (2006) Molecular signaling mechanisms underlying epileptogenesis. Sci STKE 2006(356):re12
Ates N, Esen N, Ilbay G (1999) Absence epilepsy and regional blood–brain barrier permeability: the effects of pentylenetetrazole-induced convulsions. Pharmacol Res 39:305–310
Ziylan YZ, Lefauconnier JM, Ates N, Bernard G, Bourre JM (1992) Age-dependent alteration in regional cerebrovascular permeability during drug-induced epilepsy. Mech Ageing Dev 62:319–327
Ziylan YZ, Ates N (1989) Age-related changes in regional patterns of blood–brain barrier breakdown during epileptiform seizures induced by pentylenetetrazol. Neurosci Lett 96:179–184
Fisher RS (1989) Animals models of epilepsies. Brain Res Brain Res Rev 14:245–278
Lutsenko S, Bhattacharjee A, Hubbard AL (2010) Copper handling machinery of the brain. Metallomics 2:596–608
Gaetke LM, Chow CK (2003) Copper toxicity, oxidative stress, and antioxidant nutrients. Toxicology 189:147–163
Barnes N, Tsivkovskii R, Tsivkovskaia N, Lutsenko S (2005) The copper-transporting ATPases, Menkes and Wilson disease proteins, have distinct roles in adult and developing cerebellum. J Biol Chem 280:9640–9645
Trombley PQ, Shepherd GM (1996) Differential modulation by zinc and copper of amino acid receptors from rat olfactory bulb neurons. J Neurophysiol 76:2536–2546
Vlachová V, Zemková H, Vyklický L (1996) Copper modulation of NMDA responses in mouse and rat cultured hippocampal neurons. Eur J Neurosci 8:2257–2264
Trombley PQ, Horning MS, Blakemore LJ (1998) Carnosine modulates zinc and copper effects on amino acid receptors and synaptic transmission. Neuroreport 9:3503–3507
Prasad AN, Levin S, Rupar CA, Prasad C (2011) Menkes disease and infantile epilepsy. Brain Dev 33:866–876
Kotti T, Riekkinen PJ Sr, Miettinen R (1997) Characterization of target cells for aberrant mossy fiber collaterals in the dentate gyrus of epileptic rat. Exp Neurol 146:323–330
Longo B, Covolan L, Chadi G, Mello LE (2003) Sprouting of mossy fibers and the vacating of postsynaptic targets in the innermolecular layer of the dentate gyrus. Exp Neurol 181:57–67
Nadler JV (2003) The recurrent mossy fiber pathway of the epileptic brain. Neurochem Res 28:1649–1658
Masukawa LM, O’Connor WM, Burdette LJ, McGonigle P, Sperling MR, O’Connor MJ, Uruno K (1997) Mossy fiber reorganization and its possible physiological consequences in the dentate gyrus of epileptic humans. Adv Neurol 72:53–68
Isokawa M, Mello LE (1991) NMDA receptor-mediated excitability in dendritically deformed dentate granule cells in pilocarpine-treated rats. Neurosci Lett 129:69–73
Wuarin JP, Dudek FE (1996) Electrographic seizures and new recurrent excitatory circuits in the dentate gyrus of hippocampal slices from kainate-treated epileptic rats. J Neurosci 16:4438–4448
Lynch M, Sutula T (2000) Recurrent excitatory connectivity in the dentate gyrus of kindled and kainic acid-treated rats. J Neurophysiol 83:693–704
Dudek FE, Sutula TP (2007) Epileptogenesis in the dentate gyrus: a critical perspective. Prog Brain Res 163:755–773
Frederickson CJ, Suh SW, Silva D, Frederickson CJ, Thompson RB (2000) Importance of zinc in the central nervous system: the zinc-containing neuron. J Nutr 130:1471–1483
Cavazos JE, Golarai G, Sutula TP (1991) Mossy fiber synaptic reorganization induced by kindling: time course of development, progression, and permanence. J Neurosci 11:2795–2803
Davenport CJ, Brown WJ, Babb TL (1990) Sprouting of GABAergic and mossy fiber axons in dentate gyrus following intrahippocampal kainate in the rat. Exp Neurol 109:180–190
Shibley H, Smith BN (2002) Pilocarpine-induced status epilepticus results in mossy fiber sprouting and spontaneous seizures in C57BL/6 and CD-1 mice. Epilepsy Res 49:109–120
Proper EA, Oestreicher AB, Jansen GH, Veelen CW, van Rijen PC, Gispen WH, de Graan PN (2000) Immunohistochemical characterization of mossy fiber sprouting in the hippocampus of patients with pharmaco-resistant temporal lobe epilepsy. Brain 123:19–30
Mitsuya K, Nitta N, Suzuki F (2009) Persistent zinc depletion in the mossy fiber terminals in the intrahippocampal kainate mouse model of mesial temporal lobe epilepsy. Epilepsia 50:1979–1990
Acknowledgments
This work was partially supported by the Polish Ministry of Science and Higher Education and its grant for scientific research IUVENTUS PLUS no. JP2010005370. The research leading to these results has received funding from the European Community’s Seventh Framework Programme (FP7/2007-2013) under grant agreement no. 226716 and was realized in the frame of experimental grants DESY-D-II-20080009 EC and I-20110056 EC. The authors wish to express their appreciation to Henryk Figiel for valuable discussions and comments in the preparation of the manuscript.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Chwiej, J., Dulinska, J., Janeczko, K. et al. Variations in elemental compositions of rat hippocampal formation between acute and latent phases of pilocarpine-induced epilepsy: an X-ray fluorescence microscopy study. J Biol Inorg Chem 17, 731–739 (2012). https://doi.org/10.1007/s00775-012-0892-1
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00775-012-0892-1