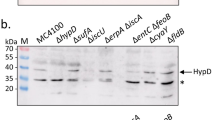
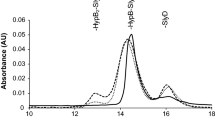
Abstract
Biosynthesis of the metallocenter in the active site of the [NiFe] hydrogenase enzyme requires the accessory protein HypB, which is a metal-binding GTPase. In this study, the interplay between the individual activities of Escherichia coli HypB was examined. The full-length protein undergoes nucleotide-responsive dimerization that is disrupted upon mutation of L242 and L246 to alanine. This mutant HypB is monomeric under all of the conditions investigated but the inability of L242A/L246A HypB to dimerize does not abolish its GTPase activity and the monomeric protein has metal-binding behavior similar to that of wild-type HypB. Furthermore, expression of L242A/L246A HypB in vivo results in hydrogenase activity that is approximately half of the activity produced by the wild-type control, suggesting that dimerization of HypB does not have a critical role in the hydrogenase maturation pathway. In contrast, the GTPase activity of HypB is modulated by metal loading of the protein. These results provide insight into the role of HypB in hydrogenase biosynthesis.




Similar content being viewed by others
Abbreviations
- AUC:
-
Analytical ultracentrifugation
- bGDP:
-
Fluorescently labelled GDP analogue BODIPY FL GDP
- GFC:
-
Gel filtration chromatography
- PAR:
-
4-(2-Pyridylazo)resorcinol
- TCEP:
-
Tris(2-carboxyethyl)phosphine
- XAS:
-
X-ray absorption spectroscopy
- WT:
-
Wild type
References
Kuchar J, Hausinger RP (2004) Chem Rev 104:509–525. doi:10.1021/cr020613p
Rosenzweig AC (2002) Chem Biol 9:673–677
Mulrooney SB, Hausinger RP (2003) FEMS Microbiol Rev 27:239–261
Li Y, Zamble DB (2009) Chem Rev 109:4617–4643. doi:10.1021/cr900010n
Kaluarachchi H, Chan Chung KC, Zamble DB (2010) Nat Prod Rep 27:681–694. doi:10.1039/b906688h
Maier T, Lottspeich F, Bock A (1995) Eur J Biochem 230:133–138
Moncrief MB, Hausinger RP (1997) J Bacteriol 179:4081–4086
Jeon WB, Cheng J, Ludden PW (2001) J Biol Chem 276:38602–38609. doi:10.1074/jbc.M104945200
Soriano A, Hausinger RP (1999) Proc Natl Acad Sci USA 96:11140–11144
Mehta N, Benoit S, Maier RJ (2003) Microb Pathog 35:229–234
Kerby RL, Ludden PW, Roberts GP (1997) J Bacteriol 179:2259–2266
Waugh R, Boxer DH (1986) Biochimie 68:157–166
Vignais PM, Billoud B, Meyer J (2001) FEMS Microbiol Rev 25:455–501
Leach MR, Zamble DB (2007) Curr Opin Chem Biol 11:159–165. doi:10.1016/j.cbpa.2007.01.011
Sawers G (1994) Antonie Van Leeuwenhoek 66:57–88
Menon NK, Chatelus CY, Dervartanian M, Wendt JC, Shanmugam KT, Peck HD Jr, Przybyla AE (1994) J Bacteriol 176:4416–4423
Sauter M, Bohm R, Bock A (1992) Mol Microbiol 6:1523–1532
Bock A, King PW, Blokesch M, Posewitz MC (2006) Adv Microb Physiol 51:1–71
Winter G, Buhrke T, Lenz O, Jones AK, Forgber M, Friedrich B (2005) FEBS Lett 579:4292–4296. doi:10.1016/j.febslet.2005.06.064
Loscher S, Zebger I, Andersen LK, Hildebrandt P, Meyer-Klaucke W, Haumann M (2005) FEBS Lett 579:4287–4291. doi:10.1016/j.febslet.2005.06.063
Theodoratou E, Huber R, Bock A (2005) Biochem Soc Trans 33:108–111. doi:10.1042/BST0330108
Leach MR, Sandal S, Leach MR, Sandal S, Sun H, Zamble DB (2005) Biochemistry 44:12229–12238. doi:10.1021/bi050993j
Chan Chung KC, Cao L, Dias AV, Pickering IJ, George GN, Zamble DB (2008) J Am Chem Soc 130:14056–14057. doi:10.1021/ja8055003
Gasper R, Scrima A, Wittinghofer A (2006) J Biol Chem 281:27492–27502. doi:10.1074/jbc.M600809200
Maier T, Jacobi A, Sauter M, Bock A (1993) J Bacteriol 175:630–635
Dias AV, Mulvihill CM, Leach MR, Pickering IJ, George GN, Zamble DB (2008) Biochemistry 47:11981–11991. doi:10.1021/bi801337x
Gill SC, von Hippel PH (1989) Anal Biochem 182:319–326
Baykov AA, Evtushenko OA, Avaeva SM (1988) Anal Biochem 171:266–270
Jacobi A, Rossmann R, Bock A (1992) Arch Microbiol 158:444–451
Leach MR, Zhang JW, Zamble DB (2007) J Biol Chem 282:16177–16186. doi:10.1074/jbc.M610834200
Ballantine SP, Boxer DH (1985) J Bacteriol 163:454–459
Zhang JW, Butland G, Greenblatt JF, Emili A, Zamble DB (2005) J Biol Chem 280:4360–4366. doi:10.1074/jbc.M411799200
Mehta N, Olson JW, Maier RJ (2003) J Bacteriol 185:726–734
Fu C, Olson JW, Maier RJ (1995) Proc Natl Acad Sci USA 92:2333–2337
Hube M, Blokesch M, Bock A (2002) J Bacteriol 184:3879–3885
Atanassova A, Zamble DB (2005) J Bacteriol 187:4689–4697. doi:10.1128/JB.187.14.4689-4697.2005
Stingl K, Schauer K, Ecobichon C, Labigne A, Lenormand P, Rousselle J-C, Namane A, De Reuse H (2008) Mol Cell Proteomics 7:2429–2441. doi:10.1074/mcp.M800160-MCP200
Leipe DD, Wolf YI, Koonin EV, Aravind L (2002) J Mol Biol 317:41–72. doi:10.1006/jmbi.2001.5378
Sydor AM, Liu J, Zamble DB (2011) J Bacteriol 193:1359–1368. doi:10.1128/JB.01333-10
Boer JL, Quiroz-Valenzuela S, Anderson KL, Hausinger RP (2010) Biochemistry 49:5859–5869. doi:10.1021/bi1004987
Jeoung JH, Giese T, Grunwald M, Dobbek H (2010) J Mol Biol 396:1165–1179. doi:10.1016/j.jmb.2009.12.062
Zambelli B, Stola M, Musiani F, De Vriendt K, Samyn B, Devreese B, Van Beeumen J, Turano P, Dikiy A, Bryant DA, Ciurli S (2005) J Biol Chem 280:4684–4695
Zambelli B, Turano P, Musiani F, Neyroz P, Ciurli S (2009) Proteins 74:222–239. doi:10.1002/prot.22205
Acknowledgments
This work was funded in part by grants from the Canadian Institutes of Health Research and the Canada Research Chairs Program. Portions of this research were carried out at the Stanford Synchrotron Radiation Lightsource, a national user facility operated by Stanford University on behalf of the US Department of Energy, Office of Basic Energy Sciences. The Stanford Synchrotron Radiation Lightsource Structural Molecular Biology Program is supported by the Department of Energy, Office of Biological and Environmental Research, and by the National Institutes of Health, National Center for Research Resources, Biomedical Technology Program.
Author information
Authors and Affiliations
Corresponding author
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Cai, F., Ngu, T.T., Kaluarachchi, H. et al. Relationship between the GTPase, metal-binding, and dimerization activities of E. coli HypB. J Biol Inorg Chem 16, 857–868 (2011). https://doi.org/10.1007/s00775-011-0782-y
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00775-011-0782-y