Abstract
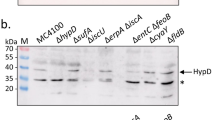
The hyp operon of Escherichia coli comprises several genes which are required for the synthesis of all three hydrogenase isoenzymes. Deletions were introduced into each of the hypA-E genes, transferred to the chromosome and the resulting mutants were analysed for hydrogenase 1, 2 and 3 activity. The products of three of the genes, hypB, hypD and hypE were found to be essential for the synthesis of all three hydrogenase isoenzymes. A defect in hypB, as previously observed, could be complemented by high nickel concentrations in the medium, whereas the effects of mutants in the other genes could not. Lesions in hypA prevented development of hydrogenase 3 activity, did not influence the level of hydrogenase 1 but led to a considerable increase in hydrogenase 2 activity although the amount of hydrogenase 2 protein was not drastically altered. Lesions in hypC, on the other hand, led to a reduction of hydrogenase 1 activity and abolished hydrogenase 3 activity. HYPA and HYPC, besides being required for hydrogenase 3 formation, therefore may have a function in modulating the activities of the three isoenzymes with respect to each other and adjusting their levels to the requirement imposed by the physiological situation. Mutations in all five hyp genes prevented the apparent processing of the large subunits of all three hydrogenase isoenzymes. It is concluded that the products of the hypA-E genes play a role in nickel incorporation into hydrogenase apoprotein and/or processing of the constituent subunits of this enzyme. The importance of their roles is also reflected in their phylogenetic conservation in distantly related organisms.
Similar content being viewed by others
References
Ballantine SP, Boxer DH (1986) Isolation and characterization of a soluble active fragment of hydrogenase isoenzyme 2 from the membranes of anaerobically grown Escherichia coli. Eur J Biochem 156: 277–284
Begg YA, Whyte JN, Haddock BA (1977) The identification of mutants of Escherichia coli deficient in formate dehydrogenase and nitrate reductase activities using dye indicator plates. FEMS Microbiol Lett 2: 47–50
Birkmann A, Böck A (1989) Characterization of a cis regulatory DNA element necessary for formate induction of the formate dehydrogenase gene (fdhF) of Escherichia coli. Mol Microbiol 3: 187–195
Birkmann A, Sawers RG, Böck A (1987a) Involvement of the ntrA gene product in the anaerobic metabolism of Escherichia coli. Mol Gen Genet 210: 535–542
Birkmann A, Zinoni F, Sawers G, Böck A (1987b) Factors affecting transcriptional regulation of the formate-hydrogen-lyase pathway of Escherichia coli. Arch Microbiol 148: 44–51
Böhrn R, Sauter M, Böck A (1990) Nucleotide sequence and expression of an operon in Escherichia coli coding for formate hydrogenlyase components. Mol Microbiol 4: 231–243
Bolivar F, Rodriguez RL, Greene PJ, Betlach MC, Heyneker HL, Boyer HW, Crosa JH, Falko S (1977) Construction and characterization of new cloning vehicles. II. A multipurpose cloning system. Gene 2: 95–113
Casadaban MJ, Cohen SN (1979) Lactose genes fused to exogenous promoters in one step using a Mu-lac bacteriophage: in vivo probe for transcriptional control sequences. Proc Natl Acad Sci USA 76: 4530–4533
Chang ACY, Cohen SN (1978) Construction and characterization of amplifiable multicopy DNA cloning vehicles derived from the P15A cryptic miniplasmid. J Bacteriol 134: 1141–1156
Eberz G, Friedrich B (1991) Three trans-acting regulatory functions control hydrogenase synthesis in Alcaligenes eutrophus. J Bacteriol 173: 1845–1854
Hamilton CM, Aldea M, Washburn BK, Babitzke P, Kushner SR (1989) New method for generating deletions and gene replacements in Escherichia coli. J Bacteriol 171: 4617–4622
Howe JG, Hershey JWB (1981) A sensitive immunoblotting method for measuring protein synthesis initiation factor levels in lysates of Escherichia coli. J Biol Chem 256: 12836–12839
Kunkel TA, Robert JD, Zakouv RA (1987) Rapid and efficient site specific mutagenesis without phenotypic selection. Methods Enzymol 154: 367–382
Lutz S, Böhm R, Beier A, Böck A (1990) Characterization of divergent NtrA-dependent promoters in the anaerobically expressed gene cluster coding for hydrogenase 3 components of Escherichia coli. Mol Microbiol 4: 13–20
Lutz S, Jacobi A, Schlensog V, Böhm R, Sawers G, Böck A (1991) Molecular characterization of an operon (hyp) necessary for the activity of the three hydrogenase isoenzymes in Escherichia coli. Mol Microbiol 5: 123–135
Menon NK, Robbins J, Peck HD, Chatelus CY, Choi E-S, Przybyla AE (1990) Cloning and sequencing of a putative Escherichia coli NiFe hydrogenase-1 operon containing six open reading frames. J Bacteriol 172: 1969–1977
Menon NK, Robbins J, Wendt JC, Shanmugam KT, Przybyla AE (1991) Mutational analysis and characterization of the Escherichia coli hya operon, which encodes [NiFe] hydrogenase 1. J Bacteriol 173: 4851–4861
Miller JH (1972) Experiments in molecular genetics. Cold Spring Harbor Laboratory Press, Cold Spring Harbor, New York
Oishi M, Cosloy SD (1972) The genetic and biochemical basis of the transformability of Escherichia coli K-12. Biochim Biophys Acta 49: 1568–1572
Przybyla AE, Robbins J, Menon N, Peck HDJr (1992) Structure/function relationships among the nickel-containing hydrogenases. FEMS Microbiol Rev 88: 109–136
Rey L, Murillo J, Hidalgo E, Palacios JM, Hernando Y, Ruiz-Argüeso T (1991) Sequencing and organisation of a gene cluster required for the synthesis of an active hydrogenase in Rhizobium leguminosarum. Abstract: Third International Conference on Molecular Biology of Hydrogenases, Troia 1991, pp 63–64
Richaud P, Colbeau A, Toussaint B, Vignais PM (1991) Identification and sequence analysis of the hupR1 gene, which encodes a response regulator of the NtrC family required for hydrogenase expression in Rhodobacter capsulatus. J Bacteriol 173: 5928–5932
Rossmann R, Sawers G, Böck A (1991) Mechanism of regulation of the formate-hydrogenlyase pathway by oxygen, nitrate and pH: definition of the formate regulon. Mol Microbiol 5: 2807–2814
Sauter M, Böhm R, Böck A (1992) Mutational analysis of the operon (hyc) determining hydrogenase 3 formation in E. coli. Mol Microbiol 6: 1523–1532
Sawers RG, Ballantine SP, Boxer DH (1985) Differential expression of hydrogenase isoenzymes in Escherichia coli K12: evidence for a third isoenzyme. J Bacteriol 164: 1324–1331
Sawers RG, Jamieson DJ, Higgins CF, Boxer DH (1986) Characterization and physiological roles of membrane-bound hydrogenase isoenzymes from Salmonella typhimurium. J Bacteriol 168: 398–404
Schlensog V, Böck A (1990) Identification and sequence analysis of the gene encoding the transcriptional activator of the formate hydrogenlyase system of E. coli. Mol Microbiol 4: 1319–1327
Schmid G, Böck A (1984) Immunoblotting analysis of ribosomal proteins from archaebacteria. Syst Appl Microbiol 5: 1–10
Vignais PM, Colbeau A, Richard P, Caballero J, Toussaint B, Elster C, Delphin C, Cauvin B (1991) Identification of a cluster of genes involved in the synthesis of the NiFe hydrogenase in the photosynthetic bacterium Rhodobacter capsulatus. Abstract: Third International Conference of Molecular Biology of Hydrogenases, Troia 1991, pp 63–64
Waugh R, Boxer DH (1986) Pleiotropic hydrogenase mutants of Escherichia coli K-12: growth in the presence of nickel can restore hydrogenase activity. Biochimie 68: 157–166
Whitaker JR, Granum PE (1980) An absolute method for protein determination based on difference in absorbance at 235 and 280 nm. Anal Biochem 109: 156–159
Winans SC, Elledge SJ, Krueger JH, Walker GC (1985) Site-directed insertion and deletion mutagenesis with cloned fragments in Escherichia coli. J Bacteriol 161: 1219–1221
Wu L-F, Navarro C, Mandrand-Berthelot M-A (1991) The hydC region contains a multicistronic operon (nik) involved in nickel transport in E. coli. Gene 107: 37–42
Xu H-W, Wall JD (1991) Clustering of genes necessary for hydrogen oxidation in Rhodobacter capsulatus. J Bacteriol 173: 2401–2405
Yanisch-Perron C, Vieira J, Messing J (1985) Improved M13 phage cloning vectors and host strains: nucleotide sequences of the M13mp18 and pUC19 vectors. Gene 33: 103–119
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Jacobi, A., Rossmann, R. & Böck, A. The hyp operon gene products are required for the maturation of catalytically active hydrogenase isoenzymes in Escherichia coli . Arch. Microbiol. 158, 444–451 (1992). https://doi.org/10.1007/BF00276307
Received:
Accepted:
Issue Date:
DOI: https://doi.org/10.1007/BF00276307