Abstract
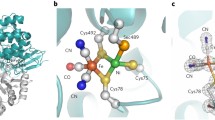
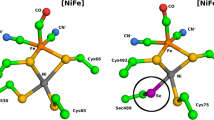
The study of Ni–Fe–Se hydrogenases is interesting from the basic research point of view because their active site is a clear example of how nature regulates the catalytic function of an enzyme by the change of a single residue, in this case a cysteine, which is replaced by a selenocysteine. Most hydrogenases are inhibited by CO and O2. In this work we studied these inhibition processes for the Ni–Fe–Se hydrogenase from Desulfovibrio vulgaris Hildenborough by combining catalytic activity measurements, followed by mass spectrometry or chronoamperometry, with Fourier transform IR spectroscopy experiments. The results show that the CO inhibitor binds to Ni in both conformations of the active site of this hydrogenase in a way similar to that in standard Ni–Fe hydrogenases, although in one of the CO-inhibited conformations the active site of the Ni–Fe–Se hydrogenase is more protected against the attack by O2. The inhibition of the Ni–Fe–Se hydrogenase activity by O2 could be explained by oxidation of the terminal cysteine ligand of the active-site Ni, instead of the direct attack of O2 on the bridging site between Ni and Fe.







Similar content being viewed by others
References
Fontecilla-Camps JC, Volbeda A, Cavazza C, Nicolet Y (2007) Chem Rev 107:4273–4303
Garcin E, Vernede X, Hatchikian EC, Volbeda A, Frey M, Fontecilla-Camps JC (1999) Structure 7:557–566
He SH, Teixeira M, LeGall J, Patil DS, Moura I, Moura JJG, Dervartanian DV, Huynh BH, Peck HD (1989) J Biol Chem 264:2678–2682
Sorgenfrei O, Duin EC, Klein A, Albracht SPJ (1996) J Biol Chem 271:23799–23806
Eidsness MK, Scott RA, Prickril BC, Dervartanian DV, LeGall J, Moura I, Moura JJG, Peck HD (1989) Proc Natl Acad Sci USA 86:147–151
Fauque G, Berlier Y, Czechowski M, Dimon B, Lespinat PA, LeGall JA (1987) J Ind Microbiol 2:15–23
Wang CP, Franco R, Moura JJG, Moura I, Day EP (1992) J Biol Chem 267:7378–7380
Vignais PM, Cournac L, Hatchikian EC, Elsen S, Serebryakova L, Zorin N, Dimon B (2002) Int J Hydrogen Energy 27:1441–1448
Valente FAA, Almeida CC, Pacheco I, Carita J, Saraiva LM, Pereira IAC (2006) J Bacteriol 188:3228–3235
Lee CM, Chuang YL, Chiang CY, Lee GH, Liaw WF (2006) Inorg Chem 45:10895–10904
Song LC, Zeng GH, Mei SZ, Lou SX, Hu QM (2006) Organometallics 25:3468–3473
Parkin A, Goldet G, Cavazza C, Fontecilla-Camps JC, Armstrong FA (2008) J Am Chem Soc 130:13410–13416
Reisner E, Powell DJ, Cavazza C, Fontecilla-Camps JC, Armstrong FA (2009) J Am Chem Soc 131:18457–18466
De Lacey AL, Gutiérrez-Sánchez C, Fernández VM, Pacheco I, Pereira IAC (2008) J Biol Inorg Chem 13:1315–1320
Bagley KA, Duin EC, Roseboom W, Albracht SPJ, Woodruff WH (1995) Biochemistry 34:5527–5535
De Lacey AL, Hatchikian EC, Volbeda A, Frey M, Fontecilla-Camps JC, Fernandez VM (1997) J Am Chem Soc 119:7181–7189
De Lacey AL, Fernandez VM, Rousset M (2005) Coord Chem Rev 249:1596–1608
Liebgott PP, Leroux F, Burlat B, Dementin S, Baffert C, Lautier T, Fourmond V, Ceccaldi P, Cavazza C, Meynial-Salles I, Soucaille P, Fontecilla-Camps JC, Guigliarelli B, Bertrand P, Rousset M, Leger C (2010) Nat Chem Biol 6:63–70
Goldet G, Brandmayr C, Stripp ST, Happe T, Cavazza C, Fontecilla-Camps JC, Armstrong FA (2009) J Am Chem Soc 131:14979–14989
Lemon BJ, Peters JW (1999) Biochemistry 38:12969–12973
Ogata H, Mizoguchi Y, Mizuno N, Miki K, Adachi S, Yasuoka N, Yagi T, Yamauchi O, Hirota S, Higuchi Y (2002) J Am Chem Soc 124:11628–11635
Volbeda A, Martin L, Cavazza C, Matho M, Faber BW, Roseboom W, Albracht SPJ, Garcin E, Rousset M, Fontecilla-Camps JC (2005) J Biol Inorg Chem 10:239–249
Vanderzwaan JW, Coremans JMCC, Bouwens ECM, Albracht SPJ (1990) Biochim Biophys Acta 1041:101–110
Carepo M, Tierney DL, Brondino CD, Yang TC, Pamplona A, Telser J, Moura I, Moura JJG, Hoffman BM (2002) J Am Chem Soc 124:281–286
Bagley KA, Van Garderen CJ, Chen M, Duin EC, Albracht SPJ, Woodruff WH (1994) Biochemistry 33:9229–9236
De Lacey AL, Stadler C, Fernandez VM, Hatchikian EC, Fan HJ, Li SH, Hall MB (2002) J Biol Inorg Chem 7:318–326
Pandelia ME, Ogata H, Currell LJ, Flores M, Lubitz W (2010) Biochim Biophys Acta 1797:304–313
Valente FMA, Oliveira ASF, Gnadt N, Pacheco I, Coelho AV, Xavier AV, Teixeira M, Soares CM, Pereira IAC (2005) J Biol Inorg Chem 10:667–682
Rüdiger O, Gutiérrez-Sánchez C, Olea D, Pereira IAC, Vélez M, Fernández VM, De Lacey AL (2010) Electroanalysis 22:776–783
Berlier Y, Fauque GD, Legall J, Choi ES, Peck HD, Lespinat PA (1987) Biochem Biophys Res Commun 146:147–153
Vincent KA, Parkin A, Armstrong FA (2007) Chem Rev 107:4366–4413
Leger C, Bertrand P (2008) Chem Rev 108:2379–2438
Nakamoto K (1997) Infrared and Raman spectra of inorganic and coordination compounds, part B, 5th edn. Wiley, New York, pp 126–148
Lubitz W, Reijerse E, van Gastel M (2007) Chem Rev 107:4331–4365
Marques MC, Coelho R, De Lacey AL, Pereira IAC, Matias PM (2010) J Mol Biol 396:893–907
Grapperhaus CA, Darensbourg MY (1998) Acc Chem Res 31:451–459
Reddie KG, Carroll KS (2008) Curr Opin Chem Biol 12:746–754
Liu TB, Li B, Singleton ML, Hall MB, Darensbourg MY (2009) J Am Chem Soc 131:8296–8307
Acknowledgments
This work was supported by the Ministerio de Educacion y Ciencia (project CTQ2009-12649, Spain), by a research grant (PTDC/BIA-PRO/70429/2006) funded by Fundação para a Ciência e Tecnologia (FCT, MCES, Portugal) and the FEDER program, and by a Luso-Spanish Joint Action funded by CRUP (Conselho de Reitores das Universidades Portuguesas, Portugal) and the Ministerio de Educación y Ciencia (HP2007-0112, Spain). We thank Pedro Matias for discussions and critical reading of the manuscript.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Gutiérrez-Sánchez, C., Rüdiger, O., Fernández, V.M. et al. Interaction of the active site of the Ni–Fe–Se hydrogenase from Desulfovibrio vulgaris Hildenborough with carbon monoxide and oxygen inhibitors. J Biol Inorg Chem 15, 1285–1292 (2010). https://doi.org/10.1007/s00775-010-0686-2
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00775-010-0686-2