Abstract
The hollow sphere-shaped 24-meric ferritin can store large amounts of iron as a ferrihydrite-like mineral core. In all subunits of homomeric ferritins and in catalytically active subunits of heteromeric ferritins a diiron binding site is found that is commonly addressed as the ferroxidase center (FC). The FC is involved in the catalytic Fe(II) oxidation by the protein; however, structural differences among different ferritins may be linked to different mechanisms of iron oxidation. Non-heme ferritins are generally believed to operate by the so-called substrate FC model in which the FC cycles by filling with Fe(II), oxidizing the iron, and donating labile Fe(III)–O–Fe(III) units to the cavity. In contrast, the heme-containing bacterial ferritin from Escherichia coli has been proposed to carry a stable FC that indirectly catalyzes Fe(II) oxidation by electron transfer from a core that oxidizes Fe(II). Here, we put forth yet another mechanism for the non-heme archaeal 24-meric ferritin from Pyrococcus furiosus in which a stable iron-containing FC acts as a catalytic center for the oxidation of Fe(II), which is subsequently transferred to a core that is not involved in Fe(II)-oxidation catalysis. The proposal is based on optical spectroscopy and steady-state kinetic measurements of iron oxidation and dioxygen consumption by apoferritin and by ferritin preloaded with different amounts of iron. Oxidation of the first 48 Fe(II) added to apoferritin is spectrally and kinetically different from subsequent iron oxidation and this is interpreted to reflect FC building followed by FC-catalyzed core formation.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Ferritin is a conserved protein that can be found in many forms of life, e.g., animals, plants, and microorganisms [1–4]. The function of ferritin appears to be essential since, e.g., deletion of its gene leads to apoptosis and death in mice embryonic cells [5]. Its quaternary structure consists of 24 subunits that assemble into a hollow spherical shape with octahedral (4-3-2) symmetry and with an outer and an inner diameter of 12 and 8 nm, respectively.
In mammals, ferritin consists of two subunits, H (“heavy”) and L (“light”) chains with molecular masses of approximately 21 and 19 kDa, respectively. It has been suggested that L subunits are catalytically inactive since no diiron nuclear center has been found in their structure, whereas H subunits are catalytically active and directly involved in the iron oxidation process [6, 7]. In amphibian red cells, three types of subunits (H, M, and L) form hetropolymeric ferritins [8]. It appears that M subunits have functional properties similar to those of H subunits. In bacterial, archaeal, and plant ferritins, all 24 subunits are identical.
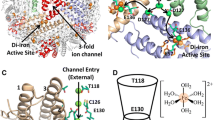
The ferritin of the hyperthermophilic archaeal anaerobe Pyrococcus furiosus (PfFtn) has 24 identical subunits, and each subunit has a diiron ferroxidase center (FC). The structure of the FC of PfFtn, based on crystallographic studies [9], is outlined in Fig. 1 including a third iron: site C. The third iron site has been found in other ferritin proteins, including Archaeoglobus fulgidus ferritin [10] and Escherichia coli ferritin A (EcFtnA) [11, 12].
The ferroxidase center of Pyrococcus furiosus ferritin including a third iron site, site C. Site C is located inside the protein shell. The figure was prepared with PyMOL and ChemDraw using coordinates from iron-soaked crystals [Protein Data Bank (PDB) entry 2jd7] [8]
It is generally believed that the FC is a so-called substrate active site, where iron is oxidized and released into the cavity to form a core [3, 13–23], and is thus functionally different from the catalytic diiron center of a similar structure found in oxygenases and in ribonucleotide reductase [24]. However, Baaghil et al. [25] have suggested that in E. coli bacterioferritin (EcBFR) Fe3+ in the FC is a stable cofactor over the time course of catalysis and acquires electrons from a core that has catalytic activity. This proposal has very recently found support in a structural study of EcBFR, in which also a novel Fe2+ binding site (named “Fe2+ internal”) was identified and proposed to act in electron transfer from the core to the FC [26]. Furthermore, Tatur and Hagen [27] found that the FC in PfFtn is a stable prosthetic group in reversible redox titrations.
On the basis of the substrate active site model, three main reactions have been proposed for the in vitro iron mineralization process by ferritin with dioxygen as an oxidant (reactions 1–3) [22, 23, 28–30].
It has been suggested that reaction 1 occurs in the FC, where two ferrous ions are oxidized and released to the core, and that it occurs at all Fe2+ to ferritin ratios. In the second reaction, ferrous ions are oxidized by hydrogen peroxide that is produced in the first reaction. Reaction 2 occurs for intermediate levels of iron loading. The third reaction has been proposed to be dominant when Fe2+ is added to apoferritin in ratios higher than 200 Fe2+ per ferritin in one step. It has been hypothesized that at high Fe2+ to ferritin ratios a core is formed which itself contributes to the oxidation of iron with a stoichiometry of four Fe2+ ions per oxygen molecule.
In the present work we used a combination of UV–vis spectroscopy and oxygen uptake measurements to study the PfFtn iron mineralization process and the functioning of the FC. Recombinant PfFtn has some practical added value as a model protein for studying the in vitro iron mineralization process: it is extremely stable [no significant loss of Fe2+-oxidation activity is observed after 10 h boiling at 100 °C] and it is highly overexpressed heterologously (25% of soluble protein in E. coli), leading to an easy purification procedure [31]. The 24-mer is homomeric, and its X-ray structure has been determined [9].
Materials and methods
Chemicals
All chemicals were purchased from Sigma-Aldrich. and were reagent grade. Bovine liver catalase (19,900 U mg−1) was also obtained from Sigma-Aldrich.
Protein, expression, and purification
Escherichia coli cells that contain the structural gene of PfFtn in vector pET24a(+) [31] were used. Cells were grown using terrific broth medium at 37 °C and 200 rpm. After 2 h, production of PfFtn was induced by addition of 1 mM isopropyl β-d-thiogalactopyranoside. Eight hours after induction, cells were harvested using centrifugation, resuspended in 10 mM tris(hydroxymethyl)aminomethane (Tris) buffer, pH 8, and broken using a cell disrupter (Constant Systems), operating at a pressure of 1.3 kbar, followed by one-step centrifugation to obtain a cell-free extract as the supernatant, which was subjected to boiling at 100 °C for 20 min. PfFtn was obtained after centrifugation and removal of the pellet containing denatured E. coli proteins. Purified protein was concentrated to 10 ml and extensively washed by sequential dilution and concentration steps with a total of 1 l of 10 mM Tris buffer.
Apoferritin preparation
Apoferritin was prepared using the method of Bauminger et al. [32] and Dawson et al. [33] under strict anaerobic conditions using an overpressure of 5.0-quality nitrogen gas. PfFtn was washed with 10 mM dithionite in Tris buffer using a 50-ml Amicon cell concentrator system with a 30-kDa filter (Millipore). Finally, the protein solution was incubated with sodium dithionite overnight at 4 °C. Free Fe2+ ions were then chelated using 2 ml of 0.5 M anaerobic solution of 2,2′-bipyridyl, followed by extensive dialysis against working buffer. The buffer was changed to 100 mM 3-(N-morpholino)propanesulfonic acid (MOPS), pH 7. The protein concentration was determined with the bicinchoninic acid assay reagent (Pierce) using bovine serum albumin as a standard.
Determination of iron content
The iron content of apoferritin was determined colorimetrically at 593 nm as the ferene complex using an Ultraspec 2100 pro spectrophotometer (GE Healthcare) as described previously [34]. Apoferritin contained less than one iron per ferritin (24-mer).
Initial-rate measurements
The steady-state kinetics of iron mineralization was followed using a fiber-optics spectrophotometer (Avantes). Initial rates were measured at different wavelengths; however, the results for the iron mineralization kinetics are reported at 315 nm unless otherwise mentioned. Spectra were recorded from approximately 5 s after the addition of an anaerobic solution of ferrous sulfate. Kinetic measurements were carried out in 100 mM MOPS, pH 7.0, using 1-ml glass cuvettes with a path length of 1 cm. The specific activity (defined as micromoles of Fe3+ formed per minute per milligram of 24-meric ferritin) was determined from the initial rate measured at 315 nm using an extinction coefficient of 2.5 mM−1 cm−1.
Preparation of Fe2+ solutions
A solution of distilled water/HCl, pH 1.25, was made anaerobic using wetted argon gas. This solution was added to the ferrous sulfate in an anaerobic glove box (less than 3 ppm O2, Coy Laboratory Products). For each titration the appropriate amount of ferrous sulfate (1–10 μl) was added to the protein solution to reach an appropriate Fe2+ to ferritin ratio.
Oxygen-consumption measurements
Oxygen consumption was measured in 100 mM MOPS buffer, pH 7.0, at ambient temperature (about 22 °C) using a Clark electrode. The setup was described by Pouvreau et al. [35]. Anaerobic ferrous sulfate was added (1–10 μl) with a gastight syringe after addition of ferritin. The volume of the cell was 2 ml. For the experiments in the presence of catalase, 200 μl of a 3 mg ml−1 stock solution of catalase was added to the cell prior to the addition of an anaerobic solution of ferrous sulfate.
Results
Iron oxidation studies
Apoferritin was loaded with 0–2,000 Fe2+ per ferritin (PfFtn can maximally accumulate about 2,700 Fe2+ per ferritin [31]) and samples were incubated at room temperature aerobically at least for 3 h and then overnight at 4 °C. The extinction coefficient at 315 nm was found to be constant over the full range of iron loading: ε 315 = 2.5 mM−1 cm−1 (Fig. 2).
Determination of the molar extinction coefficient at 315 nm for P. furiosus ferritin as a function of iron loading. a Low-iron plot: Fe2+ to ferritin ratio in the range 0–50. The protein concentration was 1.65 μM. b High-iron plot: Fe2+ to ferritin ratio in the range 0–2,000. The protein concentration was 0.105 μM
To determine the effect of background iron oxidation (i.e., not catalyzed by ferritin) in the initial-rate measurements, the specific activity of apoferritin and the background activity were measured and compared for a wide range of Fe2+ concentrations. The results indicate that the background oxidation of iron by dissolved dioxygen is negligible for an Fe2+ concentration below 1 mM, and with 0.3 μM ferritin no precipitation of Fe2+ is observed. For an Fe2+ concentration between 1 and 3 mM, some precipitation of Fe3+ develops during the ferritin assay on a time scale of minutes, and at an Fe2+ concentration higher than 3 mM the background oxidation is considerable and precipitation of iron even in the presence of ferritin is fast. The contribution of this background oxidation to the absorption at 315 nm typically increases exponentially up to saturation of the photomultiplier (Fig. S1a). Therefore, measurements at an Fe2+ concentration higher than 3 mM are considered not reliable and all subsequent experiments were done at an Fe2+ concentration lower than 1 mM. The specific activity of apoferritin for Fe2+ concentrations in the range 0.03–3 mM (Fe2+ to ferritin ratio 100–10,000) is shown in Fig. 3. As reported previously [31], an initial phase suggesting cooperativity was observed in the activity versus Fe2+ concentration plot (Fig. 3). The molecular nature of the cooperativity has not been established. No indication of zero-order kinetics is found up to 3 mM ferrous ion. A fit to the Hill equation [13, 16] affords a Hill coefficient of 1.55 ± 0.15, suggesting positive cooperativity for Fe2+ to ferritin ratios less than about 300, whereas K M and V max cannot be determined.
To study the role of the FC in iron oxidation, apoferritin was aerobically preloaded with various Fe2+ to ferritin ratios in the range 0–900. Preloaded samples were incubated at room temperature for at least 3 h and then overnight at 4 °C. Subsequently, a progress curve was measured spectroscopically at 315 nm after a further addition of 48 Fe2+ per ferritin to the samples under air. As an example, the progress curves for apoferritin, a ten Fe2+ per ferritin preloaded sample, a 20 Fe2+ per ferritin preloaded sample, and a 100 Fe2+ per ferritin preloaded sample are shown in Fig. 4a.
Comparison of the specific iron-oxidation activity of P. furiosus apoferritin with that of aerobically Fe2+-preloaded ferritin. The initial substrate concentration in all cases was 48 Fe2+ per ferritin. a Progress curves for apoferritin, a ten Fe2+ per ferritin preloaded sample, a 20 Fe2+ per ferritin preloaded sample, and a 100 Fe2+ per ferritin preloaded sample at 315 nm. The solid lines represent single-exponential or double-exponential fits. b Deconvolution into two processes of the specific activity versus the amount of Fe2+ that was preloaded aerobically to apoferritin: a process with high specific activity that decreases from apoferritin to 48 Fe2+ per ferritin preloaded sample and a process with low specific activity that develops from zero to a maximum for 48 Fe2+ per ferritin preloaded sample and that remains constant for preloaded samples with Fe2+ to ferritin ratios higher than 48. The protein concentration was 0.33 μM
The progress curves for apoferritin and for preloaded samples with more than 48 Fe2+ per ferritin showed a single exponential phase, whereas those for samples preloaded with 4, 10, and 20 Fe2+ per ferritin showed two independent phases, a fast phase and a slow phase (Fig. 4a). The data were fitted to a double-exponential curve:
in which T 1 and T 2 are time constants of the first and the second exponential, respectively. The complete set of progress curves was globally fitted with single values for T 1 and for T 2. It was observed that the preexponential factor m decreases from a maximum for apoferritin to zero for samples preloaded with Fe2+ to ferritin ratios of more than about 48, whereas the factor n increases from zero for apoferritin to a maximum for samples preloaded with Fe2+ to ferritin ratios of more than about 48. The decrease in the fast phase and the increase in the slow phase are proportional to the number of FCs that were filled by aerobic preloading of apoferritin. The specific activity for the addition of 48 Fe2+ to samples preloaded with Fe2+ to ferritin ratios greater than 48 is constant within experimental error (i.e., the specific activity is independent of the core size). Also, when 100 Fe2+ are added to samples preloaded with 100, 300, and 500 Fe2+ per ferritin, the specific activity is similar for all preloaded samples: 0.10 ± 0.02 U mg−1 (Table 1).
Progress curves were measured spectroscopically at several wavelengths for three sequential additions of aliquots of an anaerobic solution of ferrous sulfate to apoferritin, each addition corresponding to 48 Fe2+ per ferritin. The patterns monitored at 315 and 408 nm are shown in Fig. 5.
The progress curve at 315 nm shows that the first 48 Fe2+ per ferritin are kinetically different from any equal amounts of iron added later. For the first addition the absorbance at 408 nm initially increases but then falls back to zero within 10 min; however, for any subsequent addition of the same amount of iron the absorbance increases gradually and levels off upon substrate depletion.
Oxygen-consumption studies
The role of the FC in the iron-oxidation activity can also be studied by monitoring the second in vitro reactant: molecular oxygen. Aliquots of an anaerobic solution of ferrous sulfate were added to an aerobic solution of apoferritin in six steps, each step corresponding to 48 Fe2+ per ferritin. The dioxygen-consumption curve, measured amperometrically, is shown in Fig. 6.
A single-exponential curve could be fitted to each step (not shown), and the thus determined initial dioxygen uptake rates and dioxygen to Fe2+ ratios (consumed dioxygen over oxidized iron) are presented in Table 2.
In the absence of catalase, a stoichiometry of two irons per oxygen molecule is found for the first addition of 48 Fe2+ per ferritin to apoferritin, which is attributed to the reaction at the FC and suggests production of hydrogen peroxide as a byproduct of iron oxidation by ferritin (reaction 1) [22, 36]. When 48 Fe2+ per ferritin were added to apoferritin in the presence of catalase, the dioxygen to Fe2+ ratio decreased to 0.41 ± 0.02, and the initial dioxygen uptake rate was 0.3 ± 0.01 µM s−1, i.e., half of that when catalase was absent. A similar decrease in initial dioxygen uptake rate was observed for subsequent steps of adding 48 Fe2+ per ferritin to ferritin in the presence of catalase. Upon the addition of catalase, 25% of the consumed dioxygen was recovered, a number that is similar to what has been reported for H-subunit ferritin from bullfrog [37] but different from the observation that catalase can recover 100% of dioxygen for horse spleen ferritin and human ferritin [22]. The decrease in stoichiometry and initial dioxygen uptake rate in the presence of catalase strongly suggests that hydrogen peroxide is a byproduct of iron mineralization by PfFtn.
In the absence of catalase, the initial dioxygen uptake rates and the dioxygen to Fe2+ ratios gradually decrease to 0.15 ± 0.02 µM s−1 and 0.28 dioxygen per Fe2+, respectively, for the sixth step (Table 2). Because Fe2+ was added in subsequent additions with short time intervals, it is likely that some of the Fe2+ was oxidized by hydrogen peroxide that had been produced in previous steps. The decrease in stoichiometry from 1:2 dioxygen to Fe2+ to 1:3.5 dioxygen to Fe2+ can be explained as the result of reactions 1 and 2; considering that less than 50% of Fe2+ will be oxidized by hydrogen peroxide that is produced by previous steps, and more iron will be processed by PfFtn.
To prevent oxidation of Fe2+ by hydrogen peroxide, apoferritin was preloaded with 100, 500, and 900 Fe2+ per ferritin under air and preloaded samples were incubated at room temperature for at least 3 h and subsequently overnight at 4 °C to provide ample time for the essentially complete disproportionation of the hydrogen peroxide prior to subsequent iron addition. Then, an aliquot of 48 Fe2+ per ferritin was added to each sample and oxygen consumption was measured. The initial dioxygen uptake rates and the dioxygen to Fe2+ ratios are presented in Table 3.
The results presented in Table 3 show that the initial dioxygen uptake rate of apoferritin is faster than that of preloaded samples, but the initial dioxygen uptake rates of all preloaded samples are identical within experimental error. Apparently, apoferritin does not regenerate its initial activity after the first addition of 48 Fe2+ per ferritin. Whenever an aliquot of 48 Fe2+ per ferritin is added after a sufficiently long time interval, the number of Fe2+ oxidized by one oxygen molecule is close to two: dioxygen to Fe2+ ratio 0.47 ± 0.01 (Table 3). That the initial oxygen uptake rate of all preloaded samples is constant within experimental error suggests that oxidation of Fe2+ is independent of the degree of preloading, i.e., independent of the core size.
To check for possible catalytic activity of the core, indicated by a stoichiometry of four Fe2+ oxidized per dioxygen consumed (as proposed in [22, 23]), an aliquot of 300 Fe2+ per ferritin was added in one step to apoferritin and also to samples preloaded with 100, 500, and 900 Fe2+ per ferritin. Preloaded samples were incubated at room temperature for at least 3 h, followed by overnight incubation at 4 °C. The amount of dioxygen consumed by each sample is given in Table 3. Apoferritin and preloaded samples consume the same amount of dioxygen per added iron. The dioxygen to Fe2+ ratios of all samples are approximately 0.33.
Discussion
Ferritins have been believed to store iron by means of a “substrate mechanism” in which Fe2+ ions are transiently bound to FCs, where they are oxidized, and from where they are subsequently transferred to the interior of the protein to become part of a growing mineral core [3, 13–20] (Fig. 7b). However, studies on the heme-containing EcBFR have led to the proposal that this substrate mechanism may not have general validity, and that some ferritins may operate by an alternative “catalytic mechanism” in which the FC acts as a stable, redox-active prosthetic group involved in electron transfer from a core that grows by self-catalysis [25] (Fig. 7c). Furthermore, the ferritin from the archaeon P. furiosus carries a FC that appears to be fully stable in reversible redox titrations [27]. We therefore decided to investigate the “catalytic mechanism” of iron uptake, proposed for EcBFR, as a working hypothesis for PfFtn to be tested in steady-state measurements of iron loading. In particular, we searched for significant differences in the characteristics of iron loading for the first 48 Fe2+ into the empty FC sites of apoferritin as compared with the FC sites of ferritin samples preloaded with various Fe2+ to ferritin ratios. The latter were then hypothesized to be catalytically and spectroscopically different as they could potentially make use of a preformed and stable FC.
Three models proposed for iron mineralization by ferritins. a Catalysis by the ferroxidase center proposed for the P. furiosus ferritin (PDB 2jd7). Ferrous ions are oxidized in binding sites near the ferroxidase center, e.g., site C, through electron transfer to a stable ferroxidase center and are then released to the cavity to form a core. b Catalysis via a substrate site illustrated for bullfrog M ferritin (PDB1mfr). Ferrous ions are oxidized in the ferroxidase center and are then released to the cavity to form a core. c Catalysis by the core proposed for Escherichia coli bacterioferritin (PDB2htn). Ferrous ions are oxidized on the surface of an active core and electrons are transferred to an oxidant such as molecular oxygen through the ferroxidase center
Since iron oxidation by ferritins is typically monitored by an increase in optical absorption at about 315 nm, the extinction coefficient “at infinite time” (i.e., after completion of binding and oxidation) was measured for a wide range of iron loadings and was found to be constant over this range of iron concentration. In other words, formation of the FC in apoferritin cannot be discerned, on the basis of the final absorbance (i.e., after complete oxidation of all Fe2+ ions) at 315 nm, from any additional core formation.
Ferrous iron is also oxidized at finite rates in aerobic aqueous solution in the absence of ferritin, and this nonbiological reaction represents an interfering background which becomes especially pronounced at higher iron concentrations (Fig. S1). For practical ferritin concentrations and ambient temperatures, it was found that iron oxidation by ferritin becomes unreliable at an Fe2+ concentration above 3 mM. This finding also implies a correction to our initial report on the specific activity of apoferritin over a wide range of Fe2+ concentration, 0–40 mM [31]. The previous conclusion that this activity is characterized by an apparent K M of about 5 mM Fe2+ should be corrected to a K M substantially larger than 2 mM (with the implication that K M may well be infinitely high).
The results of UV–vis spectroscopy rate measurements at 315 nm suggest that the first addition of 48 Fe2+ per ferritin is kinetically different from subsequent additions of the same amount of iron. It appears that oxidation of the first 48 Fe2+ per ferritin added to apoferritin occurs in the FC with a fast rate. As the number of FCs filled by preloading the apoferritin increases, the specific activity decreases, suggesting that fewer ferrous ions can be oxidized directly in the FCs and oxidation continues by a second mechanism which has a considerably lower specific activity and which becomes dominant when all the FCs are filled, i.e., in samples preloaded with 48 Fe2+ per ferritin. The specific activity of the second reaction is independent of the core size. These findings appear to be inconsistent with a “substrate mechanism” in which all iron undergoes the same fate of passing through the FC, while restoring the FC activity to its initial state after each cycle. The data, in particular those in Fig. 4, would be consistent with a rapid synthesis of the FC (the fast phase) followed by a process in which incoming Fe2+ is catalytically oxidized by the FC to become incorporated in the core, the overall process being observed as the slow phase.
Iron oxidation rate measurements at 408 nm suggest that the first addition of 48 Fe2+ per ferritin is spectrally different from any further addition of the same amount of iron. At 408 nm, for the first addition the absorbance increases rapidly and then slowly decreases over 10 min. A similar decrease in absorbance has been observed for EcFtnA at 370 nm [38]. The molecular basis for these observations is not known but may well be a temporal rearrangement in the FC and/or in the C site.
Oxygen-consumption measurements in the absence of catalase suggest that the initial dioxygen uptake rate for the second addition of 48 Fe2+ per ferritin is lower than that for the first addition, both after a short time interval and after overnight incubation of the ferritin aerobically preloaded with 100 Fe2+ per ferritin. We conclude that, whereas it has been suggested that the FCs of a number of ferritin proteins such as human H chain ferritin, horse spleen ferritin, and mouse H chain ferritin can partially regenerate their activities [22, 23], P. furiosus apoferritin is rather more similar to EcBFR [39] in that it cannot regenerate its activity exhibited with the first addition of 48 Fe2+ per ferritin. Addition of 48 Fe2+ per ferritin to apoferritin in the presence of catalase suggests that hydrogen peroxide is a byproduct of iron deposition by ferritin and that in the absence of catalase the gradual decrease in stoichiometry is due to the oxidation of some of the iron by hydrogen peroxide.
Although the initial dioxygen uptake rate of apoferritin is higher than that of preloaded samples, both apoferritin and preloaded samples eventually consume the same amount of dioxygen per oxidized Fe2+. A stoichiometry of two irons per oxygen molecule is found, as has been reported for mammalian ferritins [22–25, 40] but which is different from the stoichiometry of three to four Fe2+ per dioxygen reported for EcFtnA [38]. Whereas the observed stoichiometry of two Fe2+ oxidized per dioxygen consumed by other groups has been interpreted in favor of a “substrate active site FC model,” the results of the kinetic studies reported here suggest that the mechanism of the reaction changes from a fast oxidation in the FC to a different mechanism (see below) after the first 48 Fe2+ per ferritin have been used to build the FCs. The fact that the stoichiometry does not change from apoferritin to preloaded samples when iron is added in aliquots of 48 Fe2+ per ferritin implies that the stoichiometry of the second mechanism is the same as the stoichiometry of the oxidation of the first 48 Fe2+ per ferritin in the FCs.
In previous studies a catalytic iron-oxidation activity of the core was proposed on the basis of the observation that the number of oxygen molecules consumed per Fe2+ oxidized gradually decreases on increasing the amount of iron added to apoferritin in one step [22, 23, 28]. If this decrease is caused by the catalytic activity of the core, it is expected that addition of 300 Fe2+ per ferritin in one step to different preloaded samples with different core sizes would lead to different stoichiometries. However, within experimental error, the dioxygen to Fe2+ ratios for apoferritin and all preloaded samples are identical. The decrease in the dioxygen to Fe2+ ratio to 0.33 compared with the ratio of 0.5 for charging with only 48 Fe2+ per ferritin may well be the result of the oxidation of Fe2+ by the hydrogen peroxide that is produced during the ferritin mineralization process. A stoichiometry of 1:4 dioxygen to Fe2+ may be expected if half of the irons are oxidized by ferritin and half by hydrogen peroxide. The observed stoichiometry of 1:3 dioxygen to Fe2+ suggests that less than 50% of the Fe2+ is oxidized by hydrogen peroxide at this loading. Similar “intermediate” stoichiometries have been reported for human H chain ferritin [23]. That the stoichiometry is identical for apoferritin and preloaded samples suggest that an iron-oxidation activity of the core, previously reported for ferritins from other sources, does not apply to PfFtn. This conclusion is in agreement with the invariant kinetics of iron oxidation observed when 100 Fe2+ per ferritin were added to three different preloaded samples, i.e., samples aerobically preloaded with 100, 300, and 500 Fe2+ per ferritin.
Altogether the results suggest that the FC in PfFtn acts as a stable cofactor site. The first 48 Fe2+ per ferritin are oxidized in the FC but do not leave the center. Oxidation of subsequently added iron appears to occur by a different mechanism, namely, a catalytic reaction that has a stoichiometry similar to that of the oxidation of the first 48 Fe2+ per ferritin in the FC, but that has a lower specific activity in comparison with the specific activity of the oxidation of iron in the FC. Since the stable FC in PfFtn has previously been found to exhibit relatively similar reduction potentials for the one-electron reduction of the all-ferric form to the mixed-valence form and then to the all-ferrous form (about +0.2 and +0.05 V [27]), we propose that the dinuclear Fe3+ site in the FC accepts two subsequent electrons from transiently nearby located Fe2+ ions (possibly at the C site; Fig. 7a) and oxidizes them to Fe3+. The resulting Fe3+ ions are then released to the cavity to form a core. To complete the cycle, electrons are transferred by the Fe3+ in the FC to an external oxidant, e.g., molecular oxygen.
The model of a catalytically active, stable prosthetic FC, proposed here, is schematically depicted in Fig. 7 together with the original substrate model and with the alternative catalytic-core model developed for bacterioferritin, including the recently discovered internal Fe2+ site proposed to be involved in electron transfer between the core and the FC [26]. As for other ferritins, the iron entry pathway for PfFtn is not known and needs further investigation. The threefold channels have been suggested as possible entry sites of Fe2+ into the ferritin cavity [41–43].
Whereas the data presented here on PfFtn appear to be incompatible with the catalytic-core model, they could still be understood in the frame of a drastically modified substrate model, namely, when one allows for the paradigm change that oxidized iron in the FC is not intrinsically labile, but only becomes mobile as the result of an event, such as a transient conformational change, induced by the binding and/or oxidation of newly incoming iron. Future experiments should help to decide the specific and perhaps general applicability of each of the alternatives outlined in Fig. 7.
Abbreviations
- EcBFR:
-
Escherichia coli bacterioferritin
- EcFtnA:
-
Escherichia coli ferritin A
- FC:
-
Ferroxidase center
- MOPS:
-
3-(N-Morpholino)propanesulfonic acid
- PfFtn:
-
Pyrococcus furiosus ferritin
- Tris:
-
Tris(hydroxymethyl)aminomethane
References
Rucker P, Torti FM, Torti SV (1996) J Biol Chem 271:33352–33357
Laulhere JP, Lescure AM, Briat JF (1988) J Biol Chem 263:10289–10294
Liu X, Theil EC (2005) Acc Chem Res 38:167–175
Lewin A, Moore GR, Le Brun NE (2005) Dalton Trans 3597–3610
Ferreira C, Bucchini D, Martin ME, Levi S, Arosio P, Grandchamp B, Beaumont C (2000) J Biol Chem 275:3021–3024
Harrison PM, Arosio P (1996) Biochim Biophys Acta 1275:161–203
Tussaint L, Bertrand L, Hue L, Crichton RR, Declercq JP (2007) J Mol Biol 365:440–452
Ha Y, Shi D, Small GW, Theil EC, Allewell NM (1999) J Biol Inorg Chem 4:243–256
Tatur J, Hagen WR, Matias PM (2007) J Biol Inorg Chem 12:615–630
Johnson E, Cascio D, Sawaya MR, Gingery M, Schröder I (2005) Structure 13:637–648
Stillman TJ, Hempstead PD, Artymiuk PJ, Andrews SC, Hudson AJ, Treffry A, Guest JR, Harrison PM (2001) J Mol Biol 307:587–603
Treffry A, Zhao Z, Quail MA, Guest JR, Harrison PM (1998) J Biol Inorg Chem 3:682–688
Liu X, Theil EC (2004) Proc Natl Acad Sci USA 101:8557–8562
Theil EC, Takagi H, Small GW, He L, Tipton AR, Danger D (2000) Inorg Chim Acta 297:242–251
Waldo GS, Ling J, Sanders-Loehr J, Theil EC (1993) Science 259:796–798
Schwartz JK, Liu XS, Tosha T, Theil EC, Solomon EI (2008) J Am Chem Soc 130:9441–9450
Tosha T, Hasan MR, Theil EC (2008) Proc Natl Acad Sci USA 105:18182–18187
Hwang J, Krebs C, Huynh BH, Edmondson DE, Theil EC, Penner-Hahn JE (1993) Science 287:122–125
Jameson GNL, Jin W, Krebs C, Perreira AS, Tavares P, Liu X, Theil EC, Huynh BH (2002) Biochemistry 41:13435–13443
Zhao G, Su M, Chasteen ND (2005) J Mol Biol 352:467–477
Bou-Abdallah F, Santambrogio P, Levi S, Arosio P, Chasteen ND (2005) J Mol Biol 347:543–554
Yang X, Chen-Barrett Y, Arosio P, Chasteen ND (1998) Biochemistry 37:9743–9750
Zhao G, Bou-Abdallah F, Arosio P, Levi S, Janus-Chandler C, Chasteen ND (2003) Biochemistry 42:3142–3150
Kolberg M, Strand KR, Graff P, Andersson KK (2004) Biochim Biophys Acta 1699:1–34
Baaghil S, Lewin A, Moore GR, Le Brun NE (2003) Biochemistry 42:14047–14056
Crow A, Lawson TL, Lewin A, Moore GR, Le Brun NE (2009) J Am Chem Soc 131:6808–6813
Tatur J, Hagen WR (2005) FEBS Lett 579:4729–4732
Xu B, Chasteen ND (1991) J Biol Chem 266:19965–19970
Chasteen ND, Harrison PM (1999) J Struct Biol 126:182–194
Proulx-Curry PM, Chasteen ND (1995) Coord Chem Rev 144:347–368
Tatur J, Hagedoorn PL, Overeijnder ML, Hagen WR (2006) Extremophiles 10:139–148
Bauminger ER, Harrison PM, Hechel D, Nowik I, Treffry A (1991) Biochim Biophys Acta 1118:48–58
Dawson MC, Elliott DC, Elliott WH, Jones KM (1986) Data for biochemical research, 3rd edn. Clarendon Press, Oxford
Matias PM, Tatur J, Carrondo MA, Hagen WR (2005) Acta Crystallogr 61:503–506
Pouvreau LAM, Strampraad MJF, Van Berloo S, Kattenberg JH, de Vries S (2008) Methods Enzymol 436:97–112
Bou-Abdallah F, Lewin AC, Le Brun NE, Moore GR, Chasteen ND (2002) J Biol Chem 277:37064–37069
Waldo GS, Theil EC (1993) Biochemistry 32:13262–13269
Treffry A, Zhao Z, Quail MA, Guest JR, Harrison PM (1998) FEBS Lett 432:213–218
Yang X, Le Brun NE, Thomson AJ, Moore GR, Chasteen ND (2000) Biochemistry 39:4915–4923
Sun S, Chasteen ND (1992) J Biol Chem 267:25160–25166
Bou-Abdallah F, Zhao G, Biasiotto G, Poli M, Arosio P, Chasteen ND (2008) J Am Chem Soc 130:17801–17811
Yablonski MJ, Theil EC (1992) Biochemistry 31:9680–9684
Treffry A, Bauminger ER, Hechel D, Hodson NW, Nowik I, Yewdall SJ, Harrison PM (1993) Biochem J 296:721–728
Acknowledgments
The PfFtn producing recombinant E. coli strain was kindly provided by J. Tatur. This work was supported by the Netherlands Research School Combination, Catalysis (NRSC-C).
Open Access
This article is distributed under the terms of the Creative Commons Attribution Noncommercial License which permits any noncommercial use, distribution, and reproduction in any medium, provided the original author(s) and source are credited.
Author information
Authors and Affiliations
Corresponding author
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This is an open access article distributed under the terms of the Creative Commons Attribution Noncommercial License (https://creativecommons.org/licenses/by-nc/2.0), which permits any noncommercial use, distribution, and reproduction in any medium, provided the original author(s) and source are credited.
About this article
Cite this article
Honarmand Ebrahimi, K., Hagedoorn, PL., Jongejan, J.A. et al. Catalysis of iron core formation in Pyrococcus furiosus ferritin. J Biol Inorg Chem 14, 1265–1274 (2009). https://doi.org/10.1007/s00775-009-0571-z
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00775-009-0571-z