Abstract
Layer-by-layer assemblies of myoglobin and polystyrenesulfonate (PSS) on pyrolitic graphite have been investigated with the goal of determining the origin of the voltammetric response of these films. From the similar midpoint potential, coverage and electron transfer behavior compared with those of adsorbed free heme, it was concluded that the observed voltammetric peak is due to heme adsorbed at the electrode surface. This suggests that the interactions between the pyrolitic graphite electrode, PSS and myoglobin can result in heme release from the protein followed by heme adsorption on the electrode.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
The immobilization of redox proteins on electrodes is a topic of active research both for obtaining a better mechanistic understanding of enzyme catalysis [1] and for the development of biosensors [2]. However, for the successful immobilization of a redox protein certain challenges have to be overcome. Firstly, the interactions between the protein and the electrode should be sufficient for immobilization, but should not significantly affect the tertiary structure of the protein. Secondly, in order to obtain electron transfer between the electrode and the redox group of the protein, the distance between them should not be much greater than 14 Å, the maximum observed for electron transfer between two redox centers in proteins [3]. To overcome these challenges, a wide variety of methods using covalent, electrostatic or hydrophobic interactions on numerous electrode materials, including gold, silver, indium oxide and pyrolitic graphite, have been investigated [4].
The extent to which an immobilization method affects the protein’s properties can be probed in different ways. On gold, silver and indium oxide electrodes spectroscopic techniques such as surface-enhanced resonance Raman spectroscopy (SERRS) [5] and surface-enhanced infrared difference adsorption spectroscopy (SEIDAS) can be employed [6]. With these techniques it has, e.g., been shown that cytochrome c retains a near-native conformation on carboxylic acid terminated self-assembled monolayers [7], whereas it undergoes significant conformational changes on bare metal electrodes [5, 8, 9]. On “dark” materials such as graphite spectroscopic measurements are difficult and therefore one has to rely largely on indirect electrochemical evidence. This includes the comparison of properties such as midpoint potential and enzymatic activity with those of the protein in solution [10–12] and the determination of whether the observed coverage and electron transfer behavior are typical of a protein [13, 14]. Using this kind of indirect evidence, we have shown that myoglobin (Mb) in films of didodecyldimethylammonium bromide (DDAB) on pyrolitic graphite can undergo heme release under certain conditions [15, 16], although our conclusions were disputed by Guto and Rusling [17]. Another case in which partial or complete heme removal from Mb has been suggested is for Mb in agarose hydrogel films in ionic liquids [18].
In the present work we investigated layer-by-layer assemblies [19] of Mb and polystyrenesulfonate (PSS) on pyrolitic graphite [20]. It has been claimed that there is fast direct electron transfer between the electrode and the protein molecules in these assemblies and therefore these assemblies are interesting for mechanistic studies of redox enzymes and for use as biosensors. They can be made by alternately adsorbing negatively charged PSS and positively charged Mb [21–25]. Our goal was to determine whether the observed electrochemical response of these assemblies is due to the native protein or to a partially or completely denatured protein. We did this by comparing the electrochemical properties of the immobilized Mb with the properties of Mb in solution and of immobilized heme, a possible denaturation product.
Materials and methods
Materials
Horse heart Mb (purity better than 90%, Aldrich), poly(sodium styrenesulfonate) (molecular weight approximately 70,000, Aldrich), poly(diallyldimethylammonium chloride) (PDDA; molecular weight approximately 200,000–350,000, Aldrich) and hemin (98%, Fluka) were used as received. All other chemicals were pro analysi grade. Buffer solutions were prepared with sodium acetate (pH 5.5), sodium dihydrogen phosphate monohydrate (pH 6–8) and boric acid (pH 9–10) combined with concentrated solutions of hydrochloric acid or sodium hydroxide and Millipore Milli-Q water (resistivity more than 18.2 MΩ cm). The concentration of the buffer was 0.1 M in all experiments, except for the electron transfer rate constant experiments, where the buffer concentration was 3.0 M. Pyrolitic graphite (Carbone–Lorraine) was fabricated into homemade rotating ring-disk electrodes [26, 27]. The geometric surface area of the electrodes was 0.5 cm2. In the electron transfer rate constant experiments the surface area of the electrode was reduced by taping off part of the surface, in order to reduce the ohmic drop.
Electrochemical apparatus and procedures
An Autolab PGstat 20 potentiostat was used for cyclic voltammetry. A homemade three-electrode cell consisting of a platinum flag counter electrode, a Hg|Hg2SO4 reference electrode and a pyrolitic graphite rotating-disk working electrode was employed. All potentials in this paper are relative to the standard hydrogen electrode (SHE). All solutions were deaerated by purging them with argon for 15 min. All electrochemical experiments were performed at room temperature.
Preparation of {Mb–PSS} n multilayer on pyrolitic graphite
Prior to use, the pyrolitic graphite electrode was abraded using P1000 SiC sandpaper and ultrasonicated in Millipore Milli-Q water for 1 min. The electrode was dried in a N2 stream for 5 s. Subsequently, the electrode was immersed in 120 μM Mb in a 0.1 M acetate solution, pH 5.5, for 10 min. After being washed with water, the electrode was immersed in 3 mg ml−1 PSS in a 0.1 M acetate solution, pH 5.5, containing 0.5 M NaCl for 10 min. This cycle was repeated to make multilayer assemblies.
Preparation of adsorbed heme on pyrolitic graphite
A 0.5 mM hemin solution was prepared by dissolving 1.6 mg hemin in 5 ml of a 0.01 M borate solution, pH 11, since hemin only dissolves in alkaline solution. Prior to use, the pyrolitic graphite electrode was abraded using P1000 SiC sandpaper and ultrasonicated in Millipore Milli-Q water for 1 min. The electrode was dried in a N2 stream for 5 s. The electrode was immersed in the hemin solution for 10 min to saturate the surface with hemin [28, 29], after which the electrode was rinsed with water.
Preparation of a PDDA–PSS–heme–PDDA–PSS layer on pyrolitic graphite
Prior to use, the pyrolitic graphite electrode was abraded using P1000 SiC sandpaper and ultrasonicated in Millipore Milli-Q water for 1 min. The electrode was immersed in 3 mg ml−1 PDDA in 0.1 M acetate solution, pH 5.5, containing 0.5 M NaCl for 10 min. After being washed with water, the electrode was immersed in a 3 mg ml−1 PSS 0.1 M acetate solution, pH 5.5, containing 0.5 M NaCl for 10 min. Subsequently, the electrode was immersed in a 0.5 mM hemin 0.01 M borate solution, pH 11, for 30 min. This was followed by 10-min immersion in the PDDA solution and 10-min immersion in the PSS solution.
Results
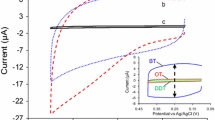
Figure 1 shows the voltammetric response of a layer-by-layer assembly of PSS and Mb on pyrolitic graphite, which was made by alternately adsorbing negatively charged PSS and positively charged Mb, both buffered at pH 5.5. The voltammetric peak corresponds to the FeIII/FeII redox transition of the protein’s heme group. The charge under the voltammetric peaks increases with the number of layers, corresponding to a coverage of approximately 2.6 × 10−10 mol cm−2 for a {Mb–PSS}6 multilayer. The midpoint potential is −0.04 ± 0.01 V versus SHE and the peak separation is 120 ± 10 mV. All these properties are in reasonable agreement with results in the literature [24] and show that we have prepared similar films.
Cyclic voltammograms of a pyrolitic graphite electrode after alternate adsorption from a 120 μM myoglobin (Mb) solution and a 3 mg ml−1 polystyrenesulfonate (PSS) solution, both buffered at pH 5.5. Voltammetry at a scan rate of 500 mV s−1 was performed in 0.1 M acetate, pH 5.5. The number of Mb–PSS layers is indicated by 1–6. SHE standard hydrogen electrode
The midpoint potential determined significantly differs from the midpoint potential of Mb in solution, which is 0.06 V versus SHE at pH 5.5 [30, 31]. This could be related to either a change in the heme environment of the protein or a local electric field, as has been reported for cytochrome c immobilized on carboxylic acid terminated self-assembled monolayers [32–35]. To obtain more insight into the effect of the immobilization, we determined the midpoint potential over a broad pH range and compared it with that of Mb in solution (Fig. 2). Figure 2 reveals differences between immobilized Mb and Mb in solution of more than 250 mV above pH 9. This is significantly more than the maximum shift of 70 mV reported between immobilized cytochrome c and cytochrome c in solution. It is unlikely that such a large shift is solely due to an electric field, suggesting that at least part of the difference is due to an altered heme environment.
Midpoint potentials of a {Mb–PSS}6 multilayer film on pyrolitic graphite (filled triangles) as a function of pH compared with those of Mb in solution (circles) [30] and adsorbed hemin on pyrolitic graphite (open triangles). Buffer 0.1 M acetate, phosphate or borate; scan rate 500 mV s−1
In previous studies on Mb–DDAB films on pyrolitic graphite we discovered that heme release can occur in these films, and that the observed electrochemical response was in fact due to isolated heme [15, 16]. To determine whether a similar process occurs in the {Mb–PSS}6 multilayer film, we compared the voltammetric response of the multilayer film with that of adsorbed hemin at pH 5.5 (Fig. 3) and with that of heme in a film of positively and negatively charged polymers. Direct comparison with a heme–PSS film is not possible, since the negatively charged PSS only adsorbs on the electrode when there is an electrostatic interaction with a positively charged polymer such as Mb. Figure 3 shows similarities and differences between the {Mb–PSS}6 multilayer and adsorbed heme. The coverage (4.0 × 10−10 mol cm−2) and midpoint potential (−0.03 ± 0.01 V vs. SHE) of adsorbed heme are comparable with those of a {Mb–PSS}6 multilayer. Especially the latter is striking, since the midpoint potential is determined by the heme environment and this is significantly different in adsorbed heme compared with Mb. To further investigate this similarity, we determined the midpoint potentials of both films over the pH 5–10 range (Fig. 2). A very close correspondence was found, which suggests that the voltammetric response of the {Mb–PSS}6 multilayer is caused by an electroactive group that closely resembles adsorbed heme. However, Fig. 3 also shows a distinctly different peak shape and lower peak separation (20 mV) for adsorbed heme than for the {Mb–PSS}6 multilayer. To determine whether this might be related to the presence of PSS in the {Mb–PSS}6 multilayer, we incorporated heme in a multilayer of positively charged PDDA and negatively charged PSS. It appeared that the presence of both charged polymers induces a peak separation of 115 mV, which is comparable to the peak separation of 120 mV observed for a {Mb–PSS}6 multilayer. This suggests that the presence of PSS can indeed cause an increased peak separation. The PDDA–PSS multilayer also induces a 30-mV shift in midpoint potential compared with adsorbed heme. Nevertheless, the pH dependence of the midpoint potential of heme incorporated in the PDDA–PSS multilayer is comparable to that of adsorbed heme (data not shown).
To learn more about the properties of a {Mb–PSS}6 multilayer, we tried to determine the electron transfer rate constant between the electrode and the heme group. This was done by measuring voltammograms at increasing scan rates. The anodic and cathodic peak potentials should start to shift with increasing scan rate, since the electron transfer cannot “keep up” with the high scan rate. From the dependence of peak separation on scan rate it is possible to calculate the electron transfer rate constants by employing Laviron’s theory [36]. However, Fig. 4 shows that in the case of a {Mb–PSS}6 multilayer, the peak separation does not shift with increasing scan rates. At 50 V s−1 the peak separation is still the same as at 500 mV s−1, namely, 130 mV. Measuring at higher scan rates is possible, but owing to a significant ohmic drop it becomes difficult to accurately determine the peak potentials; therefore, we were unable to determine the exact electron transfer rate constant. However, on the basis of the fact that no significant increase in peak separation is observed at 50 V s−1, we can deduce that the electron transfer rate constant is at least 500 s−1. Such a high electron transfer rate constant is not expected for a multilayer system where the large size of the Mb molecules implies that electrons have to tunnel over long distances in order to enable electron transfer between two protein molecules. Also, direct electron transfer between the electrode and the outer protein layers at this high rate seems unlikely, since the distance well exceeds 14 Å, the maximum for fast electron transfer between two redox centers in proteins [3]. Moreover, one would expect diffusion-controlled behavior, similar to what has been observed for layer-by-layer assemblies of cytochrome c and poly(anilinesulfonic) acid [37, 38]. Therefore, it seems more likely that the electroactive response is caused by a redox-active group which is close to the electrode, possibly adsorbed heme. We tried to determine the electron transfer rate constant for adsorbed heme, but similar to the {Mb–PSS}6 multilayer, the peak separation did not increase with increasing scan rate up to 50 V s−1 (data not shown). This implies that the electron transfer rate constant again exceeds 500 s−1. This agrees with a previous study for heme adsorbed on glassy carbon, in which a rate constant of 4.9 × 103 s−1 was found by employing potential-modulated UV–vis reflectance spectroscopy [39]. The similarity between the {Mb–PSS}6 multilayer and adsorbed heme again suggests that part of the Mb in the multilayer system undergoes heme release, which is followed by subsequent heme adsorption.
Although the poor reflection of the pyrolitic graphite electrode makes it difficult to do spectroscopy, one can gain interesting information by simple visible inspection. During the layer-by-layer formation, the color of the electrode becomes more brownish as can be seen in Fig. 5. This is due to the combination of multiple Mb layers which adsorb visible light. The darkening can therefore be used as a crude tool to determine whether the {Mb–PSS} n multilayer formation indeed occurs. In contrast, no significant darkening is observed when free heme is adsorbed on the electrode, since the monolayer coverage is too low to be visually observed.
Interestingly, when a {Mb–PSS}6 multilayer electrode is immersed in a 0.1 M borate solution of pH 9 for 10 min, the brownish color disappears from the electrode (Fig. 5). This indicates that the multilayer is desorbed from the electrode, which can be explained by the fact that at pH 9 Mb becomes negatively charged (pI = 7.35) [40] and hence the electrostatic interaction with the negatively charged PSS becomes repulsive. If the voltammetric response were due to the Mb in the multilayer, one would expect that this would also result in the disappearance of the voltammetric peaks. However, Fig. 6, which shows the voltammetric response after immersion in a pH 9.0 solution and subsequent reimmersion in a pH 5.5 solution, shows that this is not the case. The coverage decreases, but large voltammetric peaks remain. This is an indication that the voltammetric response is not due to Mb in the Mb–PSS multilayer, but instead is due to a redox-active group close to the electrode, of which only a small fraction is desorbed from the electrode when the pH is increased. Heme that is either directly adsorbed on the electrode or embedded in the first layer of PSS is the most likely candidate for this redox-active group. It is interesting to see that the peak separation decreased to 70 mV in the second measurement at pH 5.5. Probably, this is related to the fact that a large fraction of the PSS polymer desorbed from the electrode, and hence the peak separation caused by this polymer decreases.
Cyclic voltammograms of a {Mb–PSS}6 multilayer on pyrolitic graphite in a 0.1 M pH 5.5 acetate solution (thin line) and in a 0.1 M pH 9.0 borate solution (thick line). The voltammetric scan that is obtained after reimmersion in a 0.1 M pH 5.5 acetate solution is also plotted (dotted line). Scan rate 500 mV s−1
Our heme-release hypothesis would imply that free heme is formed by interactions between PSS, Mb and pyrolitic graphite. This suggests that it should be possible to obtain a voltammetric response by immersing the electrode in a mixture of PSS and Mb, and that formation of a multilayer by alternate adsorption of PSS and Mb is not necessary. Figure 7 shows that immersion in a Mb–PSS mixture indeed results in a large voltammetric peak, which is found at the same potential as for a {Mb–PSS}6 multilayer and also has a similar shape. Apart from the midpoint potential, the Mb–PSS mixture film also has a coverage (4.0 × 10−10 mol cm−2) that is comparable to the coverage of adsorbed heme. One could argue that it is possible to directly adsorb a multilayer or mixture of Mb and PSS from this solution, but this seems unlikely since there are no interactions that favor adsorption in this case. Also, the electrode does not undergo a significant darkening after immersion in the Mb–PSS mixture, whereas one would expect a darkening comparable to that of a {Mb–PSS}6 multilayer (Fig. 5), if a thick Mb–PSS film had adsorbed. Therefore, it seems more likely that the voltammetric response is due to free heme.
Cyclic voltammograms of a {Mb–PSS}6 multilayer (thin line) and a Mb–PSS mixture (dotted line) on pyrolitic graphite. Voltammetry at a scan rate of 500 mV s−1 was performed in 0.1 M acetate, pH 5.5. The Mb–PSS mixture film was made by immersing a freshly sanded pyrolitic graphite electrode in a solution of 60 μM Mb and 1.5 mg ml−1 PSS in 0.05 M pH 5.5 acetate solution with 0.25 M NaCl for 3 h
Discussion and conclusions
Our results indicate that the voltammetric response of a {Mb–PSS} n multilayer on pyrolitic graphite can be ascribed to free heme, which is either directly adsorbed on the electrode or embedded in the PSS layer. We base this on (1) the large difference in midpoint potential of a {Mb–PSS} n multilayer on pyrolitic graphite compared with that of Mb in solution, (2) the similar pH dependence of the midpoint potential of a {Mb–PSS} n multilayer on pyrolitic graphite and adsorbed heme, (3) the similar coverage of a {Mb–PSS}6 multilayer on pyrolitic graphite and adsorbed heme (4) the similar peak separation of a {Mb–PSS}6 multilayer and a PDDA–PSS–heme–PDDA–PSS film, (5) the high electron transfer rate constants of a {Mb–PSS} n multilayer, which is unlikely for electron transfer to protein molecules, but is in line with the electron transfer expected for a layer of adsorbed heme, (6) the fact that desorption of the multilayer induced by high pH does not result in the disappearance of the voltammetric peaks and (7) the fact that a large voltammetric response can be obtained by direct immersion in a Mb–PSS mixture, showing that the formation of multilayers is not necessary.
Our conclusion is in apparent disagreement with previous studies on {Mb–PSS} n layer-by-layer films on pyrolitic graphite [22–25], in which it was concluded that Mb retains a near-native conformation in the layer-by-layer films. This was concluded mainly on the basis of spectroscopic data, which consistently show that Mb retains a near-native conformation. However, these spectroscopic measurements were carried out on {Mb–PSS} n multilayers on transparent or reflective surfaces and not on pyrolitic graphite. Therefore they fail to take into account possible specific interactions between the pyrolitic graphite electrode, PSS and Mb. As we reported for Mb–DDAB films these interactions with the electrode can sometimes enhance heme release [15, 16]. The spectroscopic data therefore only show that Mb retains a near-native conformation in the absence of interactions with the electrode, corresponding to the Mb present in the layers far away from the electrode. On the other hand, our voltammetric results are a measure of the state of the protein near the electrode. Hence, the spectroscopic data are not in disagreement with our conclusion that heme release followed by heme adsorption can occur in {Mb–PSS} n multilayers on pyrolitic graphite. We therefore emphasize that we do not claim that all Mb molecules in the layer-by-layer assembly undergo heme release. Only a small fraction near the pyrolitic graphite electrode may be involved, but it is this small fraction that nevertheless determines the electrochemical response of the assembly.
References
Armstrong FA (2002) J Chem Soc Dalton Trans 661–671
Schuhmann W (2002) Rev Mol Biol 82:425
Page CC, Moser CC, Chen X, Dutton PL (1999) Nature 402:47
Armstrong FA, Wilson GS (2000) Electrochim Acta 45:2623–2645
Hildebrandt P, Stockburger M (1989) Biochemistry 28:6710
Ataka K, Heberle J (2004) J Am Chem Soc 126:9445
Hildebrandt P, Murgida DH (2002) Bioelectrochemistry 55:139
Hobara D, Niki K, Cotton TM (1998) Biospectroscopy 4:161
Wackerbarth H, Hildebrandt P (2003) ChemPhysChem 4:714–724
Elliott SJ, Hoke KR, Heffron K, Palak M, Rothery RA, Weiner JH, Armstrong FA (2004) Biochemistry 43:799
Hudson JM, Heffron K, Kotlyar V, Sher Y, Maklashina E, Cecchini G, Armstrong FA (2005) J Am Chem Soc 127:6977
Vincent KA, Parkin A, Lenz O, Albracht SPJ, Fontecilla-Camps JC, Cammack R, Friedrich B, Armstrong FA (2005) J Am Chem Soc 127:18179
de Groot MT, Evers TH, Merkx M, Koper MTM (2007) Langmuir 23:729–736
Armstrong FA, Camba R, Heering HA, Hirst J, Jeuken LJC, Jones AK, Leger C, McEvoy JP (2000) Faraday Discuss 116:191
de Groot MT, Merkx M, Koper MTM (2006) Electrochem Commum 8:999
de Groot MT, Merkx M, Koper MTM (2005) J Am Chem Soc 127:16224
Guto PM, Rusling JF (2006) Electrochem Commun 8:455–459
Brusova Z, Gorton L, Magner E (2006) Langmuir 22:11453–11455
Decher G (1997) Science 277:1232–1237
Rusling JF, Forster RJ (2003) J Colloid Interface Sci 262:1–15
Lvov Y, Ariga K, Ichinose I, Kunitake T (1995) J Am Chem Soc 117:6117–6123
Lvov Y, Ariga K, Kunitake T (1994) Chem Lett 2323–2326
Lvov YM, Lu Z, Schenkman JB, Zu X, Rusling JF (1998) J Am Chem Soc 120:4073–4080
Ma H, Hu N, Rusling JF (2000) Langmuir 16:4969–4975
Panchagnula V, Kumar CV, Rusling JF (2002) J Am Chem Soc 124:12515–12521
Van den Brink F, Visscher W, Barendrecht E (1983) J Electroanal Chem 157:283–304
Van der Plas JF, Barendrecht E (1977) Rec Trav Chim Pays Bas 96:133–136
Brown AP, Koval C, Anson FC (1976) J Electroanal Chem 72:379–387
Duong B, Arechabaleta R, Tao NJ (1998) J Electroanal Chem 447:63–69
Behlke J, Scheler W (1961) Naturwissenschaften 48:717
Brunori M, Saggese U, Rotilio GC, Antonini E, Wyman J (1971) Biochemistry 10:1604–1609
Murgida DH, Hildebrandt P (2004) Acc Chem Res 37:854–861
de Groot MT, Merkx M, Koper MTM (2007) Langmuir 23:3832–3839
Petrovic J, Clark RA, Yue H, Waldeck DH, Bowden EF (2005) Langmuir 21:6308
Fedurco M (2000) Coord Chem Rev 209:263–331
Laviron E (1979) J Electroanal Chem 101:19
Beissenhirtz MK, Kafka J, Schaefer D, Wolny M, Lisdat F (2005) Electroanalysis 17:1931–1937
Beissenhirtz MK, Scheller FW, Stocklein WFM, Kurth DG, Mohwald H, Lisdat F (2004) Angew Chem Int Ed Engl 43:4357–4360
Feng ZQ, Sagara T, Niki K (1995) Anal Chem 67:3564–3570
Radola BJ (1973) Biochim Biophys Acta 295:412–428
Acknowledgement
This work was supported by the National Research School Combination Catalysis (NRSC-C).
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
Open Access This is an open access article distributed under the terms of the Creative Commons Attribution Noncommercial License ( https://creativecommons.org/licenses/by-nc/2.0 ), which permits any noncommercial use, distribution, and reproduction in any medium, provided the original author(s) and source are credited.
About this article
Cite this article
de Groot, M.T., Merkx, M. & Koper, M.T.M. Evidence for heme release in layer-by-layer assemblies of myoglobin and polystyrenesulfonate on pyrolitic graphite. J Biol Inorg Chem 12, 761–766 (2007). https://doi.org/10.1007/s00775-007-0228-8
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00775-007-0228-8