Abstract
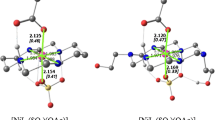
Copper(II) complexes supported by bulky tridentate ligands L1H (N,N-bis(2-quinolylmethyl)-2-phenylethylamine) and L1Ph (N,N-bis(2-quinolylmethyl)-2,2-diphenylethylamine) have been prepared and their crystal structures as well as some physicochemical properties have been explored. Each complex exhibits a square pyramidal structure containing a coordinated solvent molecule at an equatorial position and a weakly coordinated counter anion (or water) at an axial position. The copper(II) complexes reacted readily with H2O2 at a low temperature to give mononuclear hydroperoxo copper(II) complexes. Kinetics and DFT studies have suggested that, in the initial stage of the reaction, deprotonated hydrogen peroxide attacks the cupric ion, presumably at the axial position, to give a hydroperoxo copper(II) complex retaining the coordinated solvent molecule (H R·S). H R·S then loses the solvent to give a tetragonal copper(II)-hydroperoxo complex (H R), in which the –OOH group may occupy an equatorial position. The copper(II)–hydroperoxo complex H R exhibits a relatively high O–O bond stretching vibration at 900 cm−1 compared to other previously reported examples.











Similar content being viewed by others
References
Halcrow MA (2004) In: Que Jr L, Tolman WB (eds) Comprehensive Coordination Chemistry II. Elsevier, Oxford, pp 395–436
Mirica LM, Ottenwaelder X, Stack TDP (2004) Chem Rev 104:1013–1045
Lewis EA, Tolman WB (2004) Chem Rev 104:1047–1076
Klinman JP (1996) Chem Rev 96:2541–2561
Chen P, Solomon EI (2004) J Am Chem Soc 126:4991–5000
Francisco WA, Wille G, Smith AJ, Merkler DJ, Klinman JP (2004) J Am Chem Soc 126:13168–13169
Prigge ST, Eipper BA, Mains RE, Amzel LM (2004) Science 304:864–867
Fujisawa K, Tanaka M, Morooka Y, Kitajima N (1994) J Am Chem Soc 116:12079–12080
Chen P, Root DE, Campochiaro C, Fujisawa K, Solomon EI (2003) J Am Chem Soc 125:466–474
Aboelella NW, Lewis EA, Reynolds AM, Brennessel WW, Cramer CJ, Tolman WB (2002) J Am Chem Soc 124:10660–10661
Aboelella NW, Kryatov SV, Gherman BF, Grennesse WW, Young VG Jr, Sarangi R, Rybak-Akinova EV, Hodgson KO, Hedman B, Solomon EI, Cramer CJ, Tolman WB (2004) J Am Chem Soc 126:16869–16911
Cramer CJ, Tolman WB, Theopold KH, Rheingold AL (2003) Proc Natl Acad Sci USA 100:3635–3640
Chaudhuri P, Hess M, Weyhermuller T, Wieghardt K (1999) Angew Chem Int Ed 38:1095–1098
Jazdzewski BA, Reynolds AM, Holland PL, Young VG, Kaderli S, Zuberbühler AD, Tolman WB (2003) J Biol Inorg Chem 8:381–393
Weitzer M, Schindler S, Brehm G, Schneider S, Hormann E, Jung B, Kaderli S, Zuberbühler AD (2003) Inorg Chem 42:1800–1806
Komiyama K, Furutachi H, Nagatomo S, Hashimoto A, Hayashi H, Fujinami S, Suzuki M, Kitagawa T (2004) Bull Chem Soc Jpn 77:59–72
Schatz M, Raab V, Foxon SP, Brehm G, Schneider S, Reiher M, Holthausen MC, Sundermeyer J, Schindler S (2004) Angew Chem Int Ed 43:4360–4363
Wada A, Harata M, Hasegawa K, Jitsukawa K, Masuda H, Mukai M, Kitagawa T, Einaga H (1998) Angew Chem Int Ed 37:798–799
Fujii T, Naito A, Yamaguchi S, Wada A, Funahashi Y, Jitsukawa K, Nagatomo S, Kitagawa T, Masuda H (2003) Chem Commun:2700–2701
Yamaguchi S, Nagatomo S, Kitagawa T, Funahashi Y, Ozawa T, Jitsukawa K, Masuda H (2003) Inorg Chem 42:6968–6970
Ohtsu H, Itoh S, Nagatomo S, Kitagawa T, Ogo S, Watanabe Y, Fukuzumi F (2001) Inorg Chem 40:3200–3207
Chen P, Fujisawa K, Solomon EI (2000) J Am Chem Soc 122:10177–10193
Kodera M, Kita T, Miura I, Nakayama N, Kawata T, Kano K, Hirota S (2001) J Am Chem Soc 123:7715–7716
Osako T, Nagatomo S, Tachi Y, Kitagawa T, Itoh S (2002) Angew Chem Int Ed 41:4325–4328
Yamaguchi S, Kumagai A, Nagatomo S, Kitagawa T, Funahashi Y, Ozawa T, Jitsukawa K, Masuda H (2005) Bull Chem Soc Jpn 78:116–124
Karlin KD, Wei N, Jung B, Kaderli S, Niklaus P, Zuberbühler AD (1993) J Am Chem Soc 115:9506–9514
Wick PK, Karlin KD, Suzuki M, Zuberbühler AD (2004) Micron 35:137–139
Kryatov SV, Taktak S, Korendovych IV, Rybak-Akimova EV, Kaizer J, Torelli S, Shan X, Mandal S, MacMurdo VL, Payeras AM, Que Jr L (2005) Inorg Chem 44:85–99
Armarego WLF, Perrin DD (1996) Purification of laboratory chemicals. Butterworth-Heinemann, Oxford
Schmider HL, Becke AD (1998) J Chem Phys 108:9624–9631
Stevens WJ, Krauss M, Basch H, Jasien PG (1992) Can J Chem 70:612–629
Hehre WJ, Radom L, von Schleyer PR, Pople JA (1986) Ab initio molecular orbital theory. Wiley, New York
Cramer CJ (2004) Essentials of computational chemistry, 2nd edn. Wiley, Chichester
Addison AW, Rao TN, Reedijk J, Vanrijn J, Verschoor GC (1984) J Chem Soc Dalton Trans, pp 1349–1356
Solomon EI, Penfield KW, Wilcox DE (1983) Struct Bonding (Berlin) 53:1–57
Stahl SS, Thorman JL, Nelson RC, Kozee MA (2001) J Am Chem Soc 123:7188–7189
Acknowledgments
This work was financially supported in part by Grants-in-Aid for Scientific Research (No. 15350105 for SI) from the Ministry of Education, Culture, Sports, Science and Technology, Japan, by a Research Fellowship from the Japanese Society for the Promotion of Science for Young Scientists (for TO), and by the U.S. National Science Foundation (CHE-0203346 for CJC). The authors also thank Professor William B. Tolman of University of Minnesota for his helpful discussion and suggestions.
Author information
Authors and Affiliations
Corresponding authors
Electronic supplementary material
Rights and permissions
About this article
Cite this article
Osako, T., Nagatomo, S., Kitagawa, T. et al. Kinetics and DFT studies on the reaction of copper(II) complexes and H2O2 . J Biol Inorg Chem 10, 581–590 (2005). https://doi.org/10.1007/s00775-005-0005-5
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00775-005-0005-5