Abstract.
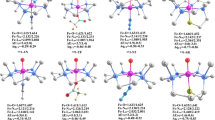
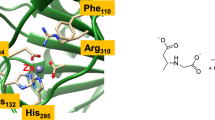
Using experimentally calibrated density functional calculations on models of the active site of soybean lipoxygenase 1 (SLO-1), insight has been obtained into the coordination flexibility of the iron active site and its molecular mechanism of catalysis. The ferrous form of SLO-1 shows a variation in coordination number in solution that is related to a weakly coordinating Asn694 ligand. From the calculations it is determined that the weak Fe-O694 bond associated with this coordination flexibility is due to a sideways tilted geometry of Asn694 that is imposed on the site by the protein. Release of this constraint (by altering the hydrogen bonding network) leads to a pure six-coordinate site. In contrast, the ferric form of the enzyme stays five-coordinate. In this case, deprotonation of a coordinated water gives a strong hydroxo donor in the cis position to Asn694, weakening the Fe-O694 bond. Hence, Asn694 is a stronger ligand to the reduced relative to the oxidized site. Using these experimentally calibrated models, the reaction energy for H-atom transfer in SLO-1 has been calculated to be about –18 kcal/mol. The observed change in coordination number going from five-coordinate in ferric to six-coordinate in ferrous SLO-1 increases the reduction potential of the iron active site. Hence, the protein adjusts the active site for optimal reactivity. Analysis of the electronic structure along the reaction coordinate shows that the H-atom transfer in SLO-1 actually corresponds to a proton-coupled electron transfer (PCET). The transferred electron does not localize on the proton, but tunnels directly from the substrate to the ferric active site in a concerted proton tunneling-electron tunneling (PTET) process. The covalently linked Fe-O-H-C bridge in the transition state lowers the energy barrier and provides an efficient superexchange pathway for this tunneling. The thermal barrier for the PTET process is estimated from the calculations to be about +15 kcal/mol including zero-point energy corrections. This corresponds to a thermal reaction rate of k therm≈1 s–1. In comparison, the rate of proton tunneling can be as high as 2×109 s–1 under these conditions. Electronic supplementary material is available if you access this article at http://dx.doi.org/10.1007/s00775-002-0415-6. On that page (frame on the left side), a link takes you directly to the supplementary material.
Similar content being viewed by others
Author information
Authors and Affiliations
Additional information
Electronic Publication
Rights and permissions
About this article
Cite this article
Lehnert, N., Solomon, E.I. Density-functional investigation on the mechanism of H-atom abstraction by lipoxygenase. J Biol Inorg Chem 8, 294–305 (2003). https://doi.org/10.1007/s00775-002-0415-6
Received:
Accepted:
Issue Date:
DOI: https://doi.org/10.1007/s00775-002-0415-6