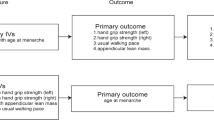
Abstract
Introduction
This study aimed to use the Mendelian randomization study method to verify the causal relationship between grip strength and bone mineral density (BMD) in different ages and different parts of the body.
Materials and methods
The analysis was based on pooled data from genome-wide association studies (GWAS). Hand grip strength (right) was used as the exposure variable and total body bone mineral density (BMD) of different age groups was used as the outcome variable. Single-nucleotide polymorphisms highly correlated with exposure variables were used as instrumental variables. The inverse variance weighted (IVW) method was used as the primary analysis method, and the Mendelian randomization Egger (MR-Egger) regression and weighted median methods were used as supplementary evidence for the IVW results. Horizontal pleiotropy and heterogeneity tests were conducted to ensure the stability of the results.
Results
Analyzing the GWAS data on osteoporosis as the outcome variable, the IVW analysis showed that osteoporosis risk was associated with decreased grip strength in the 45–60 age group and the risk of declining lumbar spine BMD was associated with decreased grip strength. However, there was no significant correlation between the risk of osteoporosis in other age groups and changes in grip strength.
Conclusion
A causal relationship exists between decreased grip strength and osteoporosis risk in people aged 45–60 years. The risk of BMD declining in the lumbar spine was associated with reduced grip strength.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Osteoporosis, a prevalent chronic metabolic bone disease [1], is marked by reduced bone mass, deterioration of bone tissue structure, and heightened fracture susceptibility. Research has indicated that among Chinese individuals aged 40, osteoporosis affected 5.0% of males and 20.6% of females [2]. Reduced BMD correlates with higher fracture vulnerability [3]. During the early twentieth century, researchers integrated BMD and clinical risk factors to create a calculator for assessing fracture risk [4].Consequently, evaluating BMD plays a vital role in diagnosing osteoporosis and forecasting fractures.
Sarcopenia is a progressive skeletal muscle disease characterized by accelerated loss of muscle mass and function, leading to adverse outcomes such as falls, functional decline, weakness, and death [5]. It primarily affects the elderly population, with muscle strength declining earlier and faster than other body composition changes [6]. Grip strength measurements are recommended for assessing sarcopenia and physical frailty in older adults due to their significant negative correlation with age [7].
The diagnosis of sarcopenia involves assessing muscle mass, strength, and physical function. Some studies have suggested a potential correlation between grip strength and BMD, indicating that grip strength is a crucial predictor of osteoporosis [8,9,10]. However, conflicting conclusions arise from a bidirectional MR study which found a clear causal relationship between osteoporosis and sarcopenia; specifically, BMD was associated with muscle mass but not muscle strength [11]. It remains unclear whether the regulation of cellular pathways explains the simultaneous or continuous relationship between skeletal muscle synthesis and bone synthesis, as well as the exact causal relationship between the two.
Due to inconsistent findings in clinical studies on the association between low grip strength and osteoporosis incidence [9, 12, 13], it is believed that this inconsistency may be due to factors related to body, disease, and environment. The occurrence of diseases is influenced by multiple factors, making it challenging for traditional epidemiological studies to distinguish causal associations with the target disease [14]. Mendelian randomization studies can establish a causal link between grip strength and BMD by eliminating confounding factors [15].
Traditional cross-sectional studies cannot establish a true causal association between exposure and outcome due to their inability to determine the order of exposure and disease occurrence or account for confounding factors. Randomized controlled trials (RCTs) face challenges such as medical ethics considerations, strict research conditions, compliance with subjects' requirements, and funding limitations that hinder their implementation. In contrast, Mendelian randomization uses genetic variation as instrumental variables to establish a causal relationship between exposure and outcome [16,17,18], providing support for the association between muscle strength and osteoporosis risk.
Handgrip strength and bone mineral density (BMD) are influenced by factors such as age, gender, height, and weight. Grip strength tends to decline after 60 years old [19], while BMD changes significantly in women aged 45–49 to 55–59 compared to men who show less reduction except between 65 and 69 years old [20]. Turning 60 is an important milestone for both women and men regarding bone mass change. Previous research suggests that hand grip strength and BMD decline synchronously after the age of 60 but are influenced by different factors. Randomized controlled trials often fail to establish true associations between pathogens and diseases, resulting in false positives. Therefore, this study aims to utilize Mendelian randomization analysis to eliminate external confounding factors and examine the correlation between grip strength and bone density across various age groups (particularly individuals aged 45–60 versus those over 60 years old).
Materials and methods
This study used grip-related phenotypes as exposure factors and total body BMD in five age groups (0–15 years, 15–30 years, 30–45 years, 45–60 years, and over 60 years), lumbar spine BMD and femoral neck BMD were used as the outcome. Three two-sample MR analyses were performed using genetic summary data from GWAS to evaluate the potential causal relationship between grip strength and BMD in five age groups and different parts of the body. Because Mendelian randomization study is a secondary analysis, the original research dataset was not separated by sex. For the reasons mentioned above, it is not possible to perform stratified analysis by sex.
Data sources
Source of muscle strength-related phenotypes
The single-nucleotide polymorphism (SNP) associated with grip strength, total body BMD, lumbar spine BMD, and femoral neck BMD in the GWAS data came from the Integrative Epidemiology Unit (IEU) OpenGWAS project (mrcieu.ac.uk) website. The website was accessed on August 15, 2023, and the study population was from Europe and included both males and females. The BMD data supporting this study's findings are openly available in [PubMed] at https://doi.org/10.1016/j.ajhg.2017.12.005 [21]. The data on hand grip strength that support the findings of this study are openly available in [Open GWAS project] at [http://gwas-api.mrcieu.ac.uk] and in [PubMed] at https://doi.org/10.1038/s41467-021-20918-w [22]. According to the relevant literature [23] of grip strength is measured by hand grip meter. The data of Lumbar spine BMD and Femoral neck BMD that support the findings of this study are openly available in [PubMed] at https://doi.org/10.1038/nature14878 [23]. According to the original text, the BMD was measured by dual-emission X-ray absorptiometry (DXA).
The sample size of the dataset was 335,842, with a total of 10,894,596 SNPs. The total body BMD sample size was 67,358, and its distribution was as follows: There were 11,807 samples in the 0–15 years age group, with 9,351,693 SNPs. The sample size in the 15–30 age group was 4,180 people with 8,509,502 SNPs. The sample size in the 30–45 age group was 10,062 people with 9,656,698 SNPs. The sample size over the age of 60 age group was 18,805 people with 10,304,110 SNPs. The sample size in the 45–60 age group was 22,504 people with 11,932,096 SNPs.
The conditions for SNP as an instrument variable
At first, instrument variables were highly correlated with the exposure, with P < 5 × 10–8 as a substantial correlation standard. Second, instrument variables have no direct relationship with outcome but only affect outcomes (no pleiotropy) through exposure, and the non-0 (P < 0.05) in this study indicated no genetic pleiotropic [24]; Third, instrumental variables were not associated with unmeasured confounding. Since the SNPs selected by the MR method follow the genetic principle that parental alleles are randomly assigned to offspring, the affected environment and acquired life span have little effect, so the study considered instrumental variables as independent of environmental factors such as socioeconomic and culture.
SNP screening rules
The SNP screening selected the meaningful SNP from the GWAS summary data of grip strength (P < 5 × 10–8, the linkage disequilibrium coefficient R2 was 0.001, and the width of the linkage disequilibrium region was 10,000 kb) to ensure that each SNP was independent of each other and exclude the influence of gene pleiotropy on the result [25]. The selected grip strength-related SNP was extracted from the GWAS summary data of total body BMD at different ages; set the minimum R2 > 0.8, and the missing SNP was replaced with an SNP with high linkage, deleting the SNP without an alternative site. The information from the above two datasets was summarized, and the SNP directly related to BMD (P < 5 × 10–8) was removed.
Analytical method
A two-sample MR analysis was performed using the instrumental SNPs to assess the causal effect of grip strength on osteoporosis risk. The summary statistics odd ratio (OR) and standard error of total body BMD at different ages enabled the indirect assessment of the causal association between hand grip strength and osteoporosis (OP) risk. In contrast, pooled comprehensive BMD measures can directly assess OP risk. We also performed the reverse MR analysis. Detailed methods for hand grip strength and BMD analysis included inverse variance weighting (IVW)-random effects, IVW-fixed meta-analysis, maximum likelihood, weighted median (WM), and MR-Egger regression, and the penalized-weighted median was applied to estimate the impact. Bonferroni correction (P-value = 0.05/5 outcomes) was used to adjust for multiple testing (P = 0.005) in this MR. All of these analyses were conducted in R V.4.1.2 using R packages of “Two-Sample MR” (https://mrcieu.github.io/TwoSampleMR/reference/clump_data.html) and P-values < 0.05 were considered statistically significant. The detailed steps are shown in the flowchart (Fig. 1).
The study performed sensitivity analyses to assess whether potential heterogeneity and pleiotropy significantly influence the results. The potential pleiotropy residual sum and outlier were evaluated by MR pleiotropy residual sum and outlier (MR-PRESSO). The Global test was used to detect the presence of heterogeneity and outliers in the instrumental variables (P < 0.05), and the Distortion test was used to examine whether there were significant differences in the results before and after removing outliers [26]. MRPRESSO analysis was conducted using the “MRPRESSO” package in R. All analyses were performed in R 4.1.2, and P < 0.05 was considered statistically significant.
Results
Instrumental variables selected SNP information
After several rounds of screening, a total of 104 grip strength-related SNPs and 514 BMD-related SNPs were included (103 SNPs from 0 to 15 years old, 102 SNPs from 15 to 30 years old, 103 SNPs from 30 to 45 years old, 103 SNPs from 45 to 60 years old, and 103 SNPs from the populations over 60 years old) after preliminary sensitivity analyses. Sixteen SNPs (rs11664336, rs2672591, rs6737767, rs11664336, rs2672591, rs6737767, rs11664336, rs2672591, rs6737767, rs74265413, rs11664336, rs2672591, rs6737767, rs11664336, rs2672591, rs6737767) were excluded, and the remaining 498 SNPs were used for MR analysis. After preliminary sensitivity analyses, 104 grip strength-related SNPs and 99 lumbar spine BMD-related SNPs were included. Four SNPs (rs11664336, rs6737767, rs74265413, rs997850) were excluded, and the remaining 99 SNPs were used for MR analysis. After preliminary sensitivity analyses, 104 grip strength-related SNPs and 99 femoral neck BMD-related SNPs were included. Four SNPs (rs11664336, rs6737767, rs74265413, rs997850) were excluded, and the remaining 95 SNPs were used for MR analysis. All the instrumental variables were strongly correlated (P < 5 × 10–8 as the screening criterion), indicating that the included instrumental variables were all effective. The bias of weak instrumental variables did not affect the causal inference results of MR analysis.
To investigate the causal relationship between total body BMD and grip strength at different ages, femoral neck BMD and grip strength, and lumbar spine BMD and grip strength, we used reverse Mendelian randomization (MR) analysis. In investigating the causal relationship between total body BMD and grip strength at different age analyses, we selected 60 single-nucleotide polymorphisms (SNP) as instrumental variables to assess the causal effects (Table 1). In investigating the causal relationship between lumbar spine BMD and grip strength, we selected 99 single-nucleotide polymorphisms (SNP) as instrumental variables to assess the causal effects (Table 2). In investigating the causal relationship between femoral neck BMD and grip strength, we selected 99 single-nucleotide polymorphisms (SNP) as instrumental variables to assess the causal effects (Table 3).
Relationship between grip strength phenotypes in different ages and different parts of body BMD
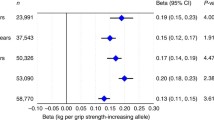
In the primary IVW analyses, hand grip strength was found to be associated with overall total body BMD [OR (95% confidence interval, CI) 1.39 (1.06–1.81), P = 0.017]. Indirectly, hand grip strength was found to be associated with the risk of OP at the age of 45–60. At the other age, hands grip strength was found to have no association with total body BMD or the risk of OP [Total body BMD(AGE:0–15): OR (95% CI) 1.102 (0.83–1.46), P = 0.49; BMD (AGE:15–30): OR (95% CI) 0.86 (0.58–1.28) P = 0.47; Total body BMD (AGE:30–45): OR (95% CI) 1.30 (0.95–1.79), P = 0.10 Total body BMD (AGE > 60) OR (95% CI) 0.99 (0.79–1.22), P = 0.89]. The result is shown in (Table 4, Fig. 2a–e, Fig. 3a–e).
In the primary IVW analyses, hand grip strength was found to be associated with Lumbar spine BMD [OR(95% confidence interval, CI) 1.31(1.06–1.60), P = 0.01]. Indirectly, hand grip strength was found to be associated with the lumbar spine BMD. However, hand grip strength was not associated with femoral neck BMD. The result is shown in (Table 4, Figs. 2f, g, 3f, g).
In reverse Mendelian randomization analyses, we did not observe any discernible causal relationship between lumbar spine BMD and femoral neck BMD as exposure factors when grip strength was utilized as the outcome variable. We did not observe any discernible causal relationship between total body BMD of different age as exposure factors when grip strength was utilized as the outcome variable (Table 5).
This conclusion is based on the analysis and comprehensive evaluation of data from many relevant studies and, therefore, has high confidence. However, due to the relatively small study sample size, further studies are needed to verify this result in the future. Furthermore, the correlation between BMD and hand grip strength may be influenced by multiple factors, e. g., bone structure, genetic factors, lifestyle, etc., so more in-depth studies are needed to explore the complexity of this issue.
Robustness
MR-Egger [24] regression method tested the possibility of horizontal pleiotropy between SNPs and the results, and the result showed no evidence of horizontal pleiotropy (P > 0.05). The funnel plots suggested that horizontal pleiotropy was not observed in any results (Fig. 4). In addition, the leave-one-out sensitivity analysis plots demonstrated that no single SNP was likely to affect causal association. Therefore, our conclusion was reliable. The MR-PRESSO results showed no outliers of horizontal pleiotropy and possible violations of causal effects in instrumental variables (Global test P < 0.05, Distortion test P = 0.011) in analyzing the relationship between total body BMD and hand grip strength at different ages. The MR-PRESSO [17] results showed that there were no outliers of horizontal pleiotropy and possible violations of causal effects in instrumental variables (Global test P < 0.01, Distortion test P < 0.01) in analyzing the relationship between lumbar spine BMD and hand grip strength. The MR-PRESSO results showed no outliers of horizontal pleiotropy and possible violations of causal effects in instrumental variables (Global test P < 0.01, Distortion test P < 0.01) in analyzing the relationship between femoral neck BMD and hand grip strength. The findings suggested that the null association between genetic predisposition to hand grip strength and total body BMD was not significantly impacted by any SNP.
In the sensitivity analysis, we found that no outliers of horizontal pleiotropy appeared in the MR-PRESSO results (Table 4) indicating that our instrumental variables did not significantly affect the authenticity and reliability of the results. These results suggest that the association between total body BMD of different ages, lumbar spine BMD, femoral neck BMD, and hand grip strength were not significantly affected by a single SNP, leading to reliable conclusions.
Discussion
This study systematically explores the potential causal relationship between grip strength and BMD from a genetic perspective, using a large population sample across different age groups. Currently, there is a lack of relevant MR studies available in the general population.
According to the molecular mechanism of muscle-related diseases, muscle-secreted factors identified in previous studies are myostatin and interleukin 6. In addition to its negative regulation of skeletal muscle, myostatin has a similar effect on bone mass [27]. When highly expressed in skeletal muscle, IL-6 can positively regulate bone mass, and the mechanism may be through the expression of the RANKL gene [28]. However, the effect of osteocytogenic factors on skeletal muscle was only recently reported. Prostaglandin E2 is a molecule produced in the osteocyte response to shear stress that can increase bone mass. Another new function of prostaglandin E2 was recently found to accelerate cell myogenic differentiation through osteocyte-like medium (PGE (2), sclerostin, and monocyte chemotaxis protein (MCP−3)) [29]. All these studies confirmed the interaction between myokine and bone factors. Recent studies have shown that the muscle-derived signal irisin is the cleaved form of fibronectin type III domain protein 5 (FNDC 5) [30], an exercise-induced myokine, which has been found to improve sarcopenia, grip strength, and muscle mass.
Meanwhile, other studies have found that irisin prevents bone loss and is essential in skeletal remodeling [31, 32]. L-β-aminoisobutyric acid (L-BAIBA) is a small molecule produced by skeletal muscle and animal experiments showed that it can increase grip strength. The results of animal experiments suggest that L-BAIBA exerts a positive regulatory effect on bone synthesis [33]. The receptor activator of nuclear factor-kappa B ligand(RANKL) signal derived from bone, which is produced by vesicles secreted by mature osteoclasts, binds to the RANKL of osteoblasts [34]. This mechanism promotes bone formation through the activation of RANKL reverse signaling, which is utilized in the treatment of osteoporosis. Additionally, it enhances muscle strength. Osteocalcin, a hormone specific to osteoblasts, facilitates mineralization of the extracellular matrix and serves as a marker for osteoblastic bone formation when measured in serum levels [35]. Undercarboxylated osteocalcin in serum independently determines hip BMD in older women [36] and exhibits muscle-protective effects in individuals without metabolic syndrome, particularly among men [37]. Furthermore, osteocalcin stimulates IL-6 synthesis indirectly influencing muscle synthesis [38]. However, the simultaneous or sequential occurrence of these two pathologies remains unclear along with their causal relationship.
According to an epidemiological study of National Health and Nutrition Examination Surveys (NHANES) 2013–2014, handgrip strength can serve as a valuable screening tool for assessing low BMD [39]. A correlation analysis investigating sarcopenia and osteoporosis revealed a significant association between presarcopenic and sarcopenic status with an elevated risk of fracture, indirectly suggesting a positive correlation between grip strength and BMD [40]. However, the current body of research primarily focuses on middle-aged and elderly populations, with limited studies conducted among other age groups. Furthermore, there is a dearth of literature exploring the relationship between handgrip strength and BMD at various anatomical sites. While one study identified a noteworthy link between grip strength and lumbar BMD, no correlation was observed with femoral neck bone density [41]. The underlying mechanism may involve parathyroid hormone (PTH) and osteocalcin, which play crucial roles in maintaining skeletal integrity and muscle growth [42]. Nevertheless, only limited studies have been conducted on this topic thus far; hence, further research is warranted.
Grip strength serves as a reliable measure of patients’ functional capacity and risk of mobility limitations, exhibiting stronger correlations with markers of frailty than chronological age, particularly among older individuals [43]. Both grip strength and bone mass decline positively correlate with advancing age, indicating a gradual deterioration in muscle strength and BMD that contributes to frailty development. Despite conflicting opinions regarding whether grip strength can accurately reflect declines in BMD, current studies demonstrate its significant predictive value for osteoporosis [44]. Due to inconsistent findings in prior studies, the potential influence of other confounding factors on the observed disparity could not be disregarded in the literature review. In this study, stratified Mendelian randomization (MR) analyses were conducted to investigate the association between grip strength and BMD across diverse age cohorts.
Based on the clinical observations, it has been determined that changes in grip strength and bone mass do not occur in the short term; rather, they represent a prolonged clinical process. Analysis of related epidemiological data suggests that the peak age for the decline in grip strength may fall between 40 and 60 years [19, 45]. After the age of 60, grip strength gradually declines. Therefore, positive results are primarily observed between the ages of 45 and 60 when considering grip strength at various age stages as exposure factors, with less significant findings in other age groups. Taking into account previous clinical epidemiological studies, it is speculated that handgrip strength experiences a significant decrease before notable decline in bone density occurs. Research examining the association between sarcopenia and osteoporosis has identified an independent contribution of handgrip strength to BMD and provides an explanation for observed changes in BMD [46].
MR studies use Mendel’s law of genetics and single-nucleotide polymorphisms (SNPs) as instrumental variables (IVs) to infer potential causal relationships between exposure factors and outcomes. This study design minimizes confounding bias and reverse causality while assuming reasonable temporality for causal associations. The results indicate a causal association between grip strength change and whole-body BMD specifically within the 45–60 age group, where grip strength changes are positively correlated with BMD changes. However, no significant association was found among other age groups. Additionally, there is a positive correlation between variations in grip strength and lumbar spine BMD changes but no significant association was identified for femoral neck BMD.
Limitations
This study systematically assessed the causal effects of grip strength and BMD across different age groups using Mendelian randomization. Although the results suggest an association between grip strength and BMD in the 45–60 age group, there are some limitations: First, only European populations were included as exposed populations and outcomes in this study, while populations of other ethnicities were not analyzed. Previous literature suggests that race may also influence research conclusions. Additionally, the relationship between sarcopenia and BMD has been found to vary based on racial backgrounds, with individuals of African descent showing the strongest correlation followed by those of European descent, and individuals of Asian ancestry having a weaker correlation [47]. An analysis of NHANES 2013–2014 database revealed a positive correlation between elevated handgrip strength and increased BMD even after adjusting for potential confounding factors such as age, gender, race, body mass index (BMI), physical activity level, smoking status, diabetes, hypertension, and high cholesterol [48]. The disparities in handgrip strength among diverse ethnic groups along with variations in the association between grip strength and BMD contribute to discrepancies in final outcomes. While this study aligns with previous research findings overall; given the numerous factors influencing handgrip strength and BMD; additional clinical data encompassing diverse populations from multiple countries is needed to further validate these observations.
Second, this study solely examined the causal relationship between grip strength and BMD within different age groups.
Third, The Mendelian randomization study, as a secondary analysis relying on existing data, must adhere to the original design's age stratification. However, due to its nature as a secondary mining and analysis of literature data, this study cannot conduct subgroup analyses based on gender, race, region, and other factors. This limitation should be acknowledged.
Recognizing these limitations, our subsequent steps involve leveraging clinical data collection and verification. Currently, we are collecting muscle strength and quality data from individuals aged over 30 years to investigate the association between grip strength and bone density. Subsequently, we plan to categorize participants into two groups based on the unique relationship between bone density and age to determine the critical value of grip strength in predicting bone density. This ongoing research is part of our endeavor.
In conclusion, the study's conclusions regarding the relationship between grip strength and BMD in people over 60 years of age have garnered increased attention. This is important due to the rising incidence of osteoporosis in this age group, especially considering that some elderly individuals are bedridden and have limited mobility. Grip strength is a simple test and a valuable predictor of osteoporosis incidence.
Within the age group of 45–60, there was an established causal relationship between grip strength and BMD. Therefore, grip strength can be utilized as one of the screening indicators for predicting osteoporosis in individuals between the ages of 45 and 60. This convenient measure allows for early intervention. However, grip strength was not associated with BMD in other age groups. Consequently, grip strength may not be a reliable predictor of BMD in individuals over 60. Therefore, when assessing the incidence of osteoporosis in individuals above 60, it is necessary to consider other factors such as muscle mass.
According to the conclusion of Mendelian randomization, we will next collect and verify clinical data to further clarify the causal relationship between the development of sarcopenia and the occurrence of osteoporosis to guide the clinical diagnosis and treatment.
Data availability
The data supporting this study's findings are available on request from the corresponding author.
References
Gregson CL, Armstrong DJ, Bowden J, Cooper C, Edwards J et al (2022) UK clinical guideline for the prevention and treatment of osteoporosis. Arch Osteoporos 17:58
Wang L, Yu W, Yin X, Cui L, Tang S et al (2021) Prevalence of osteoporosis and fracture in China: the China osteoporosis prevalence study. JAMA Netw Open 4:e2121106
Siris ES, Brenneman SK, Miller PD, Barrett-Connor E, Chen YT et al (2004) Predictive value of low bone mineral density for 1-year fracture outcomes is similar for postmenopausal women ages 50–64 and 65 and Older: results from the National Osteoporosis Risk Assessment (NORA). J Bone Miner Res 19:1215–1220
Kanis JA, Johnell O, Oden A, Johansson H, McCloskey E (2008) FRAX and the assessment of fracture probability in men and women from the UK. Osteoporosis Int 19:385–397
Morley JE (2008) Sarcopenia: diagnosis and treatment. J Nutr Health Aging 12:452–456
Westbury LD, Syddall HE, Fuggle NR, Dennison EM, Cauley JA et al (2020) Long-term rates of change in musculoskeletal aging and body composition: findings from the Health, Aging and Body Composition Study. Calcif Tissue Int 106:616–624
Porto JM, Nakaishi APM, Cangussu-Oliveira LM, FreireJúnior RC, Spilla SB et al (2019) Relationship between grip strength and global muscle strength in community-dwelling older people. Arch Gerontol Geriatr 82:273–278
Lin YH, Chen HC, Hsu NW, Chou P, Teng MMH (2021) Hand grip strength in predicting the risk of osteoporosis in Asian adults. J Bone Miner Metab 39:289–294
Schröder G, Hoth I, Flachsmeyer D, Dutzke M, Andresen JR et al (2023) Evaluation of bone density and hand grip strength in the course of drug treatment for osteoporosis: a real-world study. Orthopadie (Heidelb) 52:992–1004
McGrath R, Blackwell TL, Ensrud KE, Vincent BM, Cawthon PM (2021) The associations of handgrip strength and leg extension power asymmetry on incident recurrent falls and fractures in older men. J Gerontol A Biol Sci Med Sci 76:e221–e227
Ma XY, Liu HM, Lv WQ, Qiu C, Xiao HM et al (2022) A bi-directional Mendelian randomization study of the sarcopenia-related traits and osteoporosis. Aging (Albany NY) 14:5681–5698
Ozawa M, Hirawa N, Haze T, Haruna A, Kawano R et al (2022) The implication of calf circumference and grip strength in osteoporosis and BMD among hemodialysis patients. Clin Exp Nephrol 27:365–373
Rouzi AA, Al-Sibiani SAA, Al-Senani NS et al (2012) Independent predictors of all osteoporosis-related fractures 110healthy Saudi postmenopausal women: the CEOR study. Bone 50:713–722
Hernan MA, Robins JM (2006) Instruments for causal inference: an epidemiologist’s dream? Epidemiology 17:360–372
Emdin CA, Khera AV, Kathiresan S (2017) Mendelian randomization. JAMA 318:1925–1926
Davey Smith G, Hemani G (2014) Mendelian randomization: genetic anchors for causal inference in epidemiological studies. Hum Mol Genet 23:R89–R98
Pierce BL, Burgess S (2013) Efficient design for Mendelian randomization studies: subsample and 2-sample instrumental variable estimators. Am J Epidemiol 178:1177–1184
Burgess S, Butterworth A, Thompson SG (2013) Mendelian randomization analysis with multiple genetic variants using summarized data. Genet Epidemiol 37:658–665
Wang YC, Bohannon RW, Li X, Sindhu B, Kapellusch J (2018) Hand-grip strength: normative reference values and equations for individuals 18 to 85 years of age residing in the United States. J Orthop Sports Phys Ther 48:685–693
Yao WJ, Wu CH, Wang ST, Chang CJ, Chiu NT et al (2001) Differential changes in regional bone mineral density in healthy Chinese: age-related and sex-dependent. Calcif Tissue Int 68:330–336
Medina-Gomez C, Kemp JP, Trajanoska K, Luan J, Chesi A et al (2018) Life-course genome-wide association study meta-analysis of total body BMD and assessment of age-specific effects. Am J Hum Genet 102:88–102
Jones G, Trajanoska K, Santanasto AJ, Stringa N, Kuo CL et al (2021) Genome-wide meta-analysis of muscle weakness identifies 15 susceptibility loci in older men and women. Nat Commun 12:654
Zheng HF, Forgetta V, Hsu YH, Estrada K, Rosello-Diez A et al (2015) Whole-genome sequencing identifies EN1 as a determinant of bone density and fracture. Nature 526:112–117
Burgess S, Thompson SG (2017) Interpreting findings from Mendelian randomization using the MR-Egger method. Eur J Epidemiol 32:377–389
Xiang M, Wang Y, Gao Z, Wang J, Chen Q et al (2022) Exploring causal correlations between inflammatory cytokines and systemic lupus erythematosus: a Mendelian randomization. Front Immunol 13:985729
Verbanck M, Chen CY, Neale B, Do R (2018) Publisher Correction: Detection of widespread horizontal pleiotropy in causal relationships inferred from Mendelian randomization between complex traits and diseases. Nat Genet 50:1196
McPherron AC, Lawler AM, Lee SJ (1997) Regulation of skeletal muscle mass in mice by a new TGF-β superfamily member. Nature 387:83–90
Jonsdottir IH, Schjerling P, Ostrowski K, Asp S, Richter EA et al (2000) Muscle contractions induce interleukin-6 mRNA production in rat skeletal muscles. J Physiol 528:157–163
Iwamoto J, Takeda T, Sato Y, Yeh JK (2006) Synergistic effect of vitamin K2 and prostaglandin E2 on cancellous bone mass in hypophysectomized young rats. Calcif Tissue Int 79:318–325
Mo C, Romero-Suarez S, Bonewald L, Johnson M, Brotto M (2012) Prostaglandin E2: from clinical applications to its potential role in bone- muscle crosstalk and myogenic differentiation. Recent Pat Biotechnol 6:223–229
Guo M, Yao J, Li J, Zhang J, Wang D et al (2023) Irisin ameliorates age-associated sarcopenia and metabolic dysfunction. J Cachexia Sarcopenia Muscle 14:391–405
Kim H, Wrann CD, Jedrychowski M, Vidoni S, Kitase Y et al (2018) Irisin mediates effects on bone and fat via αV integrin receptors. Cell 175:1756–1768 (e17)
Roberts LD, Boström P, O’Sullivan JF, Schinzel RT, Lewis GD et al (2014) β-Aminoisobutyric acid induces browning of white fat and hepatic β-oxidation and is inversely correlated with cardiometabolic risk factors. Cell Metab 19:96–108
Prideaux M, Smargiassi A, Peng G, Brotto M, Robling AG et al (2023) L-BAIBA synergizes with sub-optimal mechanical loading to promote new bone formation. JBMR Plus 7:e10746
Hamoudi D, Bouredji Z, Marcadet L, Yagita H, Landry LB et al (2020) Muscle weakness and selective muscle atrophy in osteoprotegerin-deficient mice. Hum Mol Genet 29:483–494
Delmas PD, Eastell R, Garnero P, Seibel MJ, Stepan J (2000) Committee of Scientific Advisors of the International Osteoporosis Foundation. The use of biochemical markers of bone turnover in osteoporosis. Committee of Scientific Advisors of the International Osteoporosis Foundation. Osteoporos Int 11:S2–S17
Szulc P, Arlot M, Chapuy MC, Duboeuf F, Meunier PJ et al (1994) Serum undercarboxylated osteocalcin correlates with hip BMD in elderly women. J Bone Miner Res 9:1591–1595
Xiang Y, Lu W, Mao X, Zou J, Wang J et al (2023) Osteocalcin has a muscle-protective effect during weight loss in men without metabolic syndrome: a multicenter, prospective, observational study. Front Endocrinol (Lausanne) 14:1308452
Luo Y, Jiang K, He M (2020) Association between grip strength and BMD in general US population of NHANES 2013–2014. Arch Osteoporos 15:47
Jauffret C, Périchon R, Lamer A, Cortet B, Chazard E et al (2023) Association between Sarcopenia and fracture risk in a population from the UK biobank database. J Bone Miner Res 38:1422–1434
Santos LA, Lima TB, Augusti L, FranzoniLde C, Yamashiro Fda S et al (2016) Handgrip strength as a predictor of bone mineral density in outpatients with cirrhosis. J Gastroenterol Hepatol 31:229–234
Brent MB, Brüel A, Thomsen JS (2018) PTH (1–34) and growth hormone in prevention of disuse osteopenia and sarcopenia in rats. Bone 110:244–253
Jacob I, Johnson MI, Jones G, Jones A, Francis P (2022) Age-related differences in vastus lateralis muscle morphology, contractile properties, upper body grip strength, and lower extremity functional capability in healthy adults aged 18 to 70 years. BMC Geriatr 22:1–15
Bijlsma AY, Meskers MC, Molendijk M, Westendorp RG, Sipilä S et al (2013) Diagnostic measures for sarcopenia and bone mineral density. Osteoporos Int 24:2681–2691
Gómez-Campos R, Vidal Espinoza R, de Arruda M, Ronque ERV, Urra-Albornoz C et al (2023) Relationship between age and handgrip strength: proposal of reference values from infancy to senescence. Front Public Health 10:1072684
He H, Liu Y, Tian Q, Papasian CJ, Hu T et al (2016) Relationship of sarcopenia and body composition with osteoporosis. Osteoporos Int 27:473–482
Ning HT, Du Y, Zhao LJ, Tian Q, Feng H et al (2021) Racial and gender differences in the relationship between sarcopenia and BMD among older adults. Osteoporos Int 32:841–851
Wu N, Li X, Mu S, Fu Q, Ba G (2021) Handgrip strength is positively associated with bone mineral density in middle and aged adults: results from NHANES 2013–2014. Arch Osteoporos 16:121
Acknowledgements
During the major revision process, Jiabei He provides guidance on data supplementation, while Xin Li offers guidance on writing modifications. I would like to express my gratitude to Jiabei He and Xin Li for their invaluable assistance.
Funding
This work was not supported by any funding.
Author information
Authors and Affiliations
Contributions
Yingying Zhu designed the study and supervised the overall project. Kede Chi participated in data collecting and analysis. Yingying Zhu provided the statistical analysis and wrote the manuscript.
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Ethical standards
We obtained written informed consent from the patients. We were under the 1975 Helsinki Declaration and its later amendments.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
This article is published under an open access license. Please check the 'Copyright Information' section either on this page or in the PDF for details of this license and what re-use is permitted. If your intended use exceeds what is permitted by the license or if you are unable to locate the licence and re-use information, please contact the Rights and Permissions team.
About this article
Cite this article
Zhu, Y., Chi, K. & Wang, J. Mendelian randomization study on association between grip strength and BMD in different age groups. J Bone Miner Metab (2024). https://doi.org/10.1007/s00774-024-01519-1
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s00774-024-01519-1