Abstract
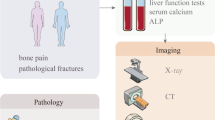
Bone is a frequent site of metastasis for multiple types of solid tumors in organs such as prostate, breast, lung, etc., accounting for significant morbidities and mortalities of afflicted patients. One of the major problems of bone metastasis is lack of biomarkers for early diagnosis and for monitoring therapeutic responses. Medical imaging modalities such as computerized tomography, magnetic resonance imaging, and radioactive isotope-based bone scans are currently standard clinical practices, yet these imaging techniques are limited to detect early lesions or to accurately monitor the metastatic disease progression during standard and/or experimental therapies. Accordingly, development of novel blood biomarkers rationalizes extensive basic research and clinical development. This review article covers the up-to-date information on protein- and cell-based biomarkers of bone metastasis that are currently used in the clinical practices and also are under development.
Similar content being viewed by others
References
Hofbauer LC, Rachner TD, Coleman RE, Jakob F (2014) Endocrine aspects of bone metastases. Lancet Diabetes Endocrinol 2:500–512. https://doi.org/10.1016/S2213-8587(13)70203-1
Mundy GR (2002) Metastasis to bone: causes, consequences and therapeutic opportunities. Nat Rev Cancer 2:584–593. https://doi.org/10.1038/nrc867
Eriksen EF, Axelrod DW, Melsen F (1994) Bone histomorphometry. Raven Press
Shiozawa Y, Pedersen EA, Havens AM et al (2011) Human prostate cancer metastases target the hematopoietic stem cell niche to establish footholds in mouse bone marrow. J Clin Invest 121:1298–1312. https://doi.org/10.1172/JCI43414
Shiozawa Y, Pienta KJ, Taichman RS (2011) Hematopoietic stem cell niche is a potential therapeutic target for bone metastatic tumors. Clin Cancer Res 17:5553–5558. https://doi.org/10.1158/1078-0432.CCR-10-2505
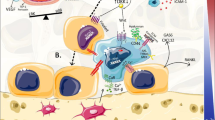
Jeong HM, Cho SW, Park SI (2016) Osteoblasts Are the Centerpiece of the Metastatic Bone Microenvironment. Endocrinol Metab 31:485–492. https://doi.org/10.3803/EnM.2016.31.4.485
Cook GJR, Azad GK, Goh V (2016) Imaging bone metastases in breast cancer: staging and response assessment. J Nucl Med 57:27S-33S. https://doi.org/10.2967/jnumed.115.157867
Goyal N, Kalra M, Soni A et al (2019) Multi-modality imaging approach to bone tumors - State-of-the art. J Clin Orthop Trauma 10:687–701. https://doi.org/10.1016/j.jcot.2019.05.022
Koizumi M, Matsumoto S, Takahashi S et al (1999) Bone metabolic markers in the evaluation of bone scan flare phenomenon in bone metastases of breast cancer. Clin Nucl Med 24:15–20. https://doi.org/10.1097/00003072-199901000-00004
Hirahata T, Ul Quraish R, Quraish AU et al (2022) Liquid biopsy: a distinctive approach to the diagnosis and prognosis of cancer. Cancer Inform 21:11769351221076062. https://doi.org/10.1177/11769351221076062
Coleman R, Costa L, Saad F et al (2011) Consensus on the utility of bone markers in the malignant bone disease setting. Crit Rev Oncol Hematol 80:411–432. https://doi.org/10.1016/j.critrevonc.2011.02.005
Coleman RE, Lipton A, Costa L et al (2013) Possible survival benefits from zoledronic acid treatment in patients with bone metastases from solid tumours and poor prognostic features-An exploratory analysis of placebo-controlled trials. J bone Oncol 2:70–76. https://doi.org/10.1016/j.jbo.2013.01.002
Tamiya M, Tokunaga S, Okada H et al (2013) Prospective study of urinary and serum cross-linked N-telopeptide of type I collagen (NTx) for diagnosis of bone metastasis in patients with lung cancer. Clin Lung Cancer 14:364–369. https://doi.org/10.1016/j.cllc.2012.11.006
Coleman RE, Major P, Lipton A et al (2005) Predictive value of bone resorption and formation markers in cancer patients with bone metastases receiving the bisphosphonate zoledronic acid. J Clin Oncol 23:4925–4935. https://doi.org/10.1200/JCO.2005.06.091
Brown JE, Cook RJ, Major P et al (2005) Bone turnover markers as predictors of skeletal complications in prostate cancer, lung cancer, and other solid tumors. J Natl Cancer Inst 97:59–69. https://doi.org/10.1093/jnci/dji002
Lara PNJ, Ely B, Quinn DI et al (2014) Serum biomarkers of bone metabolism in castration-resistant prostate cancer patients with skeletal metastases: results from SWOG 0421. J Natl Cancer Inst 106:dju013. https://doi.org/10.1093/jnci/dju013
Jung K, Lein M, Stephan C et al (2004) Comparison of 10 serum bone turnover markers in prostate carcinoma patients with bone metastatic spread: diagnostic and prognostic implications. Int J cancer 111:783–791. https://doi.org/10.1002/ijc.20314
Brown J, Rathbone E, Hinsley S et al (2018) Associations between serum bone biomarkers in early breast cancer and development of bone metastasis: results from the AZURE (BIG01/04) trial. J Natl Cancer Inst 110:871–879. https://doi.org/10.1093/jnci/djx280
Lipton A, Chapman J-AW, Demers L et al (2011) Elevated bone turnover predicts for bone metastasis in postmenopausal breast cancer: results of NCIC CTG MA.14. J Clin Oncol 29:3605–3610. https://doi.org/10.1200/JCO.2010.31.5069
Kong QQ, Sun TW, Dou QY et al (2007) Beta-CTX and ICTP act as indicators of skeletal metastasis status in male patients with non-small cell lung cancer. Int J Biol Markers 22:214–220. https://doi.org/10.5301/jbm.2008.3777
Koopmans N, de Jong IJ, Breeuwsma AJ, van der Veer E (2007) Serum bone turnover markers (PINP and ICTP) for the early detection of bone metastases in patients with prostate cancer: a longitudinal approach. J Urol 178:849–853. https://doi.org/10.1016/j.juro.2007.05.029
Wada N, Fujisaki M, Ishii S et al (2001) Evaluation of bone metabolic markers in breast cancer with bone metastasis. Breast Cancer 8:131–137. https://doi.org/10.1007/BF02967492
Lumachi F, Basso SMM, Camozzi V et al (2016) Bone turnover markers in women with early stage breast cancer who developed bone metastases. A prospective study with multivariate logistic regression analysis of accuracy. Clin Chim acta 460:227–230. https://doi.org/10.1016/j.cca.2016.07.005
Lyubimova NV, Pashkov MV, Tyulyandin SA et al (2004) Tartrate-resistant acid phosphatase as a marker of bone metastases in patients with breast cancer and prostate cancer. Bull Exp Biol Med 138:77–79. https://doi.org/10.1023/b:bebm.0000046945.95479.d6
Salminen E, Ala-Houhala M, Korpela J et al (2005) Serum tartrate-resistant acid phosphatase 5b (TRACP 5b) as a marker of skeletal changes in prostate cancer. Acta Oncol (Madr) 44:742–747. https://doi.org/10.1080/02841860500327586
Elfar GA, Ebrahim MA, Elsherbiny NM, Eissa LA (2017) Validity of Osteoprotegerin and Receptor Activator of NF-κB Ligand for the Detection of Bone Metastasis in Breast Cancer. Oncol Res 25:641–650. https://doi.org/10.3727/096504016X14768398678750
Oremek G, Sauer-Eppel H, Klepzig M (2007) Total procollagen type 1 amino-terminal propeptide (total P1NP) as a bone metastasis marker in gynecological carcinomas. Anticancer Res 27:1961–1962
Westerhuis LW, Delaere KP (1997) Diagnostic value of some biochemical bone markers for the detection of bone metastases in prostate cancer. Eur J Clin Chem Clin Biochem 35:89–94. https://doi.org/10.1515/cclm.1997.35.2.89
Brown JE, Cook RJ, Lipton A et al (2010) Prognostic factors for skeletal complications from metastatic bone disease in breast cancer. Breast Cancer Res Treat 123:767–779. https://doi.org/10.1007/s10549-010-0981-1
Du W-X, Duan S-F, Chen J-J et al (2014) Serum bone-specific alkaline phosphatase as a biomarker for osseous metastases in patients with malignant carcinomas: a systematic review and meta-analysis. J Cancer Res Ther 10:C140–C143. https://doi.org/10.4103/0973-1482.145842
Lipton A, Smith MR, Fizazi K et al (2016) Changes in bone turnover marker levels and clinical outcomes in patients with advanced cancer and bone metastases treated with bone antiresorptive agents. Clin cancer Res 22:5713–5721. https://doi.org/10.1158/1078-0432.CCR-15-3086
Zhao H, Han K-L, Wang Z-Y et al (2011) Value of C-telopeptide-cross-linked Type I collagen, osteocalcin, bone-specific alkaline phosphatase and procollagen Type I N-terminal propeptide in the diagnosis and prognosis of bone metastasis in patients with malignant tumors. Med Sci Monit 17:CR626-633. https://doi.org/10.12659/msm.882047
Arai Y, Takeuchi H, Oishi K, Yoshida O (1992) Osteocalcin: is it a useful marker of bone metastasis and response to treatment in advanced prostate cancer? Prostate 20:169–177. https://doi.org/10.1002/pros.2990200302
Terpos E, Kiagia M, Karapanagiotou EM et al (2009) The clinical significance of serum markers of bone turnover in NSCLC patients: surveillance, management and prognostic implications. Anticancer Res 29:1651–1657
Bayrak SB, Ceylan E, Serter M et al (2012) The clinical importance of bone metabolic markers in detecting bone metastasis of lung cancer. Int J Clin Oncol 17:112–118. https://doi.org/10.1007/s10147-011-0266-7
Lee K-H, Lee KJ, Kim T-Y et al (2020) Circulating Osteocalcin-positive cells as a novel diagnostic biomarker for bone metastasis in breast cancer patients. J Bone Miner Res 35:1838–1849. https://doi.org/10.1002/jbmr.4041
Larson SR, Zhang X, Dumpit R et al (2013) Characterization of osteoblastic and osteolytic proteins in prostate cancer bone metastases. Prostate 73:932–940. https://doi.org/10.1002/pros.22639
Hesse E, Schröder S, Brandt D et al (2019) Sclerostin inhibition alleviates breast cancer-induced bone metastases and muscle weakness. JCI Insight. https://doi.org/10.1172/jci.insight.125543
Rachner TD, Göbel A, Thiele S et al (2014) Dickkopf-1 is regulated by the mevalonate pathway in breast cancer. Breast cancer Res 16:R20. https://doi.org/10.1186/bcr3616
Kasoha M, Bohle RM, Seibold A et al (2018) Dickkopf-1 (Dkk1) protein expression in breast cancer with special reference to bone metastases. Clin Exp Metastasis 35:763–775. https://doi.org/10.1007/s10585-018-9937-3
Qiao R, Zhong R, Chang Q et al (2017) Serum dickkopf-1 as a clinical and prognostic factor in non-small cell lung cancer patients with bone metastases. Oncotarget 8:79469–79479. https://doi.org/10.18632/oncotarget.18446
Ramankulov A, Lein M, Kristiansen G et al (2007) Elevated plasma osteopontin as marker for distant metastases and poor survival in patients with renal cell carcinoma. J Cancer Res Clin Oncol 133:643–652. https://doi.org/10.1007/s00432-007-0215-z
Ramankulov A, Lein M, Kristiansen G et al (2007) Plasma osteopontin in comparison with bone markers as indicator of bone metastasis and survival outcome in patients with prostate cancer. Prostate 67:330–340. https://doi.org/10.1002/pros.20540
Hiraki A, Ueoka H, Bessho A et al (2002) Parathyroid hormone-related protein measured at the time of first visit is an indicator of bone metastases and survival in lung carcinoma patients with hypercalcemia. Cancer 95:1706–1713. https://doi.org/10.1002/cncr.10828
Washam CL, Byrum SD, Leitzel K et al (2013) Identification of PTHrP(12–48) as a plasma biomarker associated with breast cancer bone metastasis. Cancer Epidemiol Biomark Prev 22:972–983. https://doi.org/10.1158/1055-9965.EPI-12-1318-T
Abuzallouf S, Dayes I, Lukka H (2004) Baseline staging of newly diagnosed prostate cancer: a summary of the literature. J Urol 171:2122–2127. https://doi.org/10.1097/01.ju.0000123981.03084.06
Briganti A, Suardi N, Gallina A et al (2014) Predicting the risk of bone metastasis in prostate cancer. Cancer Treat Rev 40:3–11. https://doi.org/10.1016/j.ctrv.2013.07.001
Sutherland A, Forsyth A, Cong Y et al (2016) The role of prolactin in bone metastasis and breast cancer cell-mediated osteoclast differentiation. J Natl Cancer Inst. https://doi.org/10.1093/jnci/djv338
D’Amico L, Belisario D, Migliardi G et al (2016) C-met inhibition blocks bone metastasis development induced by renal cancer stem cells. Oncotarget 7:45525–45537. https://doi.org/10.18632/oncotarget.9997
Lee SK, Park K-K, Kim H-J et al (2017) Human antigen R-regulated CCL20 contributes to osteolytic breast cancer bone metastasis. Sci Rep 7:9610. https://doi.org/10.1038/s41598-017-09040-4
Wang J, You H, Qi J et al (2017) Autocrine and paracrine STIP1 signaling promote osteolytic bone metastasis in renal cell carcinoma. Oncotarget 8:17012–17026. https://doi.org/10.18632/oncotarget.15222
Tiedemann K, Sadvakassova G, Mikolajewicz N et al (2019) Exosomal release of L-plastin by breast cancer cells facilitates metastatic bone osteolysis. Transl Oncol 12:462–474. https://doi.org/10.1016/j.tranon.2018.11.014
Helo P, Cronin AM, Danila DC et al (2009) Circulating prostate tumor cells detected by reverse transcription-PCR in men with localized or castration-refractory prostate cancer: concordance with Cell Search assay and association with bone metastases and with survival. Clin Chem 55:765–773. https://doi.org/10.1373/clinchem.2008.117952
De Giorgi U, Valero V, Rohren E et al (2010) Circulating tumor cells and bone metastases as detected by FDG-PET/CT in patients with metastatic breast cancer. Ann Oncol 21:33–39. https://doi.org/10.1093/annonc/mdp262
Cheng M, Liu L, Yang H-S, Liu G-F (2014) Circulating tumor cells are associated with bone metastasis of lung cancer. Asian Pacif J cancer Prev 15:6369–6374. https://doi.org/10.7314/apjcp.2014.15.15.6369
Pantano F, Rossi E, Iuliani M et al (2020) Dynamic changes of receptor activator of nuclear factor-κB expression in circulating tumor cells during Denosumab predict treatment effectiveness in Metastatic Breast Cancer. Sci Rep 10:1288. https://doi.org/10.1038/s41598-020-58339-2
Rizzo FM, Vesely C, Childs A et al (2019) Circulating tumour cells and their association with bone metastases in patients with neuroendocrine tumours. Br J Cancer 120:294–300. https://doi.org/10.1038/s41416-018-0367-4
Zhu L, Loo WTY, Chow LWC (2005) Circulating tumor cells in patients with breast cancer: possible predictor of micro-metastasis in bone marrow but not in sentinel lymph nodes. Biomed Pharmacother 59:S355–S358. https://doi.org/10.1016/s0753-3322(05)80077-0
Zhu L, Loo WTY, Cheng CWN, Chow LWC (2006) Possible predictive markers related to micro-metastasis in breast cancer patients. Oncol Rep 15:1217–1223
Trapp EK, Fasching PA, Fehm T et al (2022) Does the presence of circulating tumor cells in high-risk early breast cancer patients predict the site of first metastasis-results from the adjuvant SUCCESS a trial. Cancers (Basel). https://doi.org/10.3390/cancers14163949
Iuliani M, Simonetti S, Ribelli G et al (2020) Current and emerging biomarkers predicting bone metastasis development. Front Oncol 10:789. https://doi.org/10.3389/fonc.2020.00789
Plaks V, Koopman CD, Werb Z (2013) Cancer circulating tumor cells. Science 341:1186–1188. https://doi.org/10.1126/science.1235226
Tian R, Li X, Zhang H et al (2022) Ulex Europaeus agglutinin-I-based magnetic isolation for the efficient and specific capture of SW480 circulating colorectal tumor cells. ACS Omega 7:30405–30411. https://doi.org/10.1021/acsomega.2c03702
Kolostova K, Spicka J, Matkowski R, Bobek V (2015) Isolation, primary culture, morphological and molecular characterization of circulating tumor cells in gynecological cancers. Am J Transl Res 7:1203–1213
Yoon J, Yoon H-S, Shin Y et al (2017) Ethanol-dispersed and antibody-conjugated polymer nanofibers for the selective capture and 3-dimensional culture of EpCAM-positive cells. Nanomedicine 13:1617–1625. https://doi.org/10.1016/j.nano.2017.02.015
Eghbali-Fatourechi GZ, Lamsam J, Fraser D et al (2005) Circulating osteoblast-lineage cells in humans. N Engl J Med 352:1959–1966. https://doi.org/10.1056/NEJMoa044264
Dumez B, Van Damme K, Casteleyn L (2007) Research on the socio-ethical impact of biomarker use and the communication processes in ECNIS NoE and NewGeneris IP. Int J Hyg Environ Health 210:263–265. https://doi.org/10.1016/j.ijheh.2007.01.018
Suda RK, Billings PC, Egan KP et al (2009) Circulating osteogenic precursor cells in heterotopic bone formation. Stem Cells 27:2209–2219. https://doi.org/10.1002/stem.150
De Potter P, von Weymarn C, Zografos L (1991) In vivo phosphorus 31 magnetic resonance spectroscopy of human uveal melanomas and other intraocular tumors. Am J Ophthalmol 111:276–288. https://doi.org/10.1016/s0002-9394(14)72310-4
Undale A, Srinivasan B, Drake M et al (2010) Circulating osteogenic cells: characterization and relationship to rates of bone loss in postmenopausal women. Bone 47:83–92. https://doi.org/10.1016/j.bone.2010.03.018
Rubin MR, Manavalan JS, Dempster DW et al (2011) Parathyroid hormone stimulates circulating osteogenic cells in hypoparathyroidism. J Clin Endocrinol Metab 96:176–186. https://doi.org/10.1210/jc.2009-2682
Egan KP, Duque G, Keenan MA, Pignolo RJ (2018) Circulating osteogentic precursor cells in non-hereditary heterotopic ossification. Bone 109:61–64. https://doi.org/10.1016/j.bone.2017.12.028
Johnson RW (2020) The search for a bone metastasis biomarker may have a new find: circulating osteocalcin-positive cells. J Bone Miner Res 35:1836–1837
Lee C, Whang YM, Campbell P et al (2018) Dual targeting c-met and VEGFR2 in osteoblasts suppresses growth and osteolysis of prostate cancer bone metastasis. Cancer Lett 414:205–213. https://doi.org/10.1016/j.canlet.2017.11.016
Park SI, Lee C, Sadler WD et al (2013) Parathyroid hormone-related protein drives a CD11b+Gr1+ cell-mediated positive feedback loop to support prostate cancer growth. Cancer Res 73:6574–6583. https://doi.org/10.1158/0008-5472.CAN-12-4692
Zheng Y, Chow S-O, Boernert K et al (2014) Direct crosstalk between cancer and osteoblast lineage cells fuels metastatic growth in bone via auto-amplification of IL-6 and RANKL signaling pathways. J Bone Miner Res 29:1938–1949. https://doi.org/10.1002/jbmr.2231
Swami S, Johnson J, Bettinson LA et al (2017) Prevention of breast cancer skeletal metastases with parathyroid hormone. JCI Insight. https://doi.org/10.1172/jci.insight.90874
Devignes C-S, Aslan Y, Brenot A et al (2018) HIF signaling in osteoblast-lineage cells promotes systemic breast cancer growth and metastasis in mice. Proc Natl Acad Sci USA 115:E992–E1001. https://doi.org/10.1073/pnas.1718009115
Masucci MT, Minopoli M, Carriero MV (2019) Tumor associated neutrophils their role in tumorigenesis, metastasis Prognosis and Therapy. Front Oncol 9:1146. https://doi.org/10.3389/fonc.2019.01146
Thio QCBS, Goudriaan WA, Janssen SJ et al (2018) Prognostic role of neutrophil-to-lymphocyte ratio and platelet-to-lymphocyte ratio in patients with bone metastases. Br J Cancer 119:737–743. https://doi.org/10.1038/s41416-018-0231-6
Wang S, Zhang Z, Fang F et al (2011) The neutrophil/lymphocyte ratio is an independent prognostic indicator in patients with bone metastasis. Oncol Lett 2:735–740. https://doi.org/10.3892/ol.2011.304
Caliskan B, Korkmaz AN (2016) Can Neutrophil/Lymphocyte Ratio be a Predictor for Bone Metastases of Solid Tumors? World J Nucl Med 15:196–199. https://doi.org/10.4103/1450-1147.174711
Camerer E, Qazi AA, Duong DN et al (2004) Platelets, protease-activated receptors, and fibrinogen in hematogenous metastasis. Blood 104:397–401. https://doi.org/10.1182/blood-2004-02-0434
Felding-Habermann B, Fransvea E, O’Toole TE et al (2002) Involvement of tumor cell integrin alpha v beta 3 in hematogenous metastasis of human melanoma cells. Clin Exp Metastasis 19:427–436. https://doi.org/10.1023/a:1016377114119
Bakewell SJ, Nestor P, Prasad S et al (2003) Platelet and osteoclast beta3 integrins are critical for bone metastasis. Proc Natl Acad Sci USA 100:14205–14210. https://doi.org/10.1073/pnas.2234372100
Boucharaba A, Serre C-M, Grès S et al (2004) Platelet-derived lysophosphatidic acid supports the progression of osteolytic bone metastases in breast cancer. J Clin Invest 114:1714–1725. https://doi.org/10.1172/JCI22123
Park SI, Liao J, Berry JE et al (2012) Cyclophosphamide creates a receptive microenvironment for prostate cancer skeletal metastasis. Cancer Res 72:2522–2532. https://doi.org/10.1158/0008-5472.CAN-11-2928
Lee EJ, Jung S, Park KH, Park SI (2022) Flow cytometry-based immunophenotyping of myeloid-derived suppressor cells in human breast cancer patient blood samples. J Immunol Methods 510:113348. https://doi.org/10.1016/jj.im.2022.113348
Funding
This study was supported by the National R&D Program for Cancer Control, the Ministry of Health and Welfare, the Republic of Korea (HA17C0040) and the National Research Foundation of Korea (2019R1A2C1085672, 2020R1A2C1012966 and 2022R1A6A3A01087551).
Author information
Authors and Affiliations
Corresponding authors
Ethics declarations
Conflict of interest
Sun Wook Cho is a co-founder and CEO of Cellus, Inc.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
About this article
Cite this article
Song, MK., Park, S.I. & Cho, S.W. Circulating biomarkers for diagnosis and therapeutic monitoring in bone metastasis. J Bone Miner Metab 41, 337–344 (2023). https://doi.org/10.1007/s00774-022-01396-6
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00774-022-01396-6