Abstract
Introduction
Despite the high prevalence of hypocalcemia in patients with COVID-19, very limited studies have been designed to evaluate etiologies of this disorder. This study was designed to evaluate the status of serum parameters involved in calcium metabolism in patients with COVID-19 and hypocalcemia.
Materials and methods
This cross-sectional study was conducted on 123 hospitalized patients with COVID-19. Serum concentrations of PTH, 25 (OH) D, magnesium, phosphate, and albumin were assessed and compared across three groups of moderate/severe hypocalcemia (serum total calcium < 8 mg/dl), mild hypocalcemia (8 mg/dl ≤ serum total calcium < 8.5 mg/dl) and normocalcemia (serum total calcium ≥ 8.5 mg/dl). Multivariate analyses were performed to evaluate the independent roles of serum parameters in hypocalcemia.
Results
In total, 65.9% of the patients had hypocalcemia. Vitamin D deficiency was found in 44.4% and 37.7% of moderate/severe and mild hypocalcemia cases, respectively, compared to 7.1% in the normal serum total calcium group (P = 0.003). In multivariate analysis, vitamin D deficiency was independently associated with 6.2 times higher risk of hypocalcemia (P = 0.001). Only a minority of patients with hypocalcemia had appropriately high PTH (15.1% and 14.3% in mild and moderate/severe hypocalcemia, respectively). Serum PTH was low/low-normal in 40.0% of patients with moderate/severe low-corrected calcium group. Magnesium deficiency was not associated with hypocalcemia in univariate and multivariate analysis.
Conclusion
Vitamin D deficiency plays a major role in hypocalcemia among hospitalized patients with COVID-19. Inappropriately low/low-normal serum PTH may be a contributing factor in this disorder.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Since the beginning of the COVID-19 pandemic in December 2019, other manifestations have been progressively reported in various organs in addition to the respiratory system [1]. The endocrine system is one of the targets of Coronavirus. New-onset diabetes, adrenal insufficiency, syndrome of inappropriate secretion of anti-diuretic hormone, and hypocalcemia have been reported in this disease [2,3,4].
Hypocalcemia is highly prevalent in COVID-19 [5,6,7]. Up to 80% of hospitalized patients with COVID-19 have been reported to have hypocalcemia [5]. Furthermore, hypocalcemia in COVID-19 is associated with progressive disease and poor outcomes [7, 8]. Despite being a highly prevalent disorder and its robust role in the prognosis of patients with COVID-19, very limited studies have investigated the etiologies of its occurrence.
Hypocalcemia has been reported as a prevalent disorder in critically ill patients since decades ago [9, 10]. Different mechanisms such as hypoalbuminemia, disorders of PTH secretion and action, vitamin D deficiency or increased catabolism of vitamin D, and calcium precipitation are involved in the hypocalcemia of critical illness [11,12,13].
Despite some similarities between hypocalcemia in COVID-19 and other critical illnesses, it seems that this disorder is somehow different in COVID-19. Firstly, albeit based on limited studies, hypocalcemia is more prevalent in the acute respiratory syndrome by COVID-19 infection compared to other infections [14]; secondly, hypocalcemia in COVID-19 is not restricted to severe or critical cases and the prevalence of this disorder is high even in non-severeCOVID-19 [15].
Regarding the dearth of evidence about the etiologies of hypocalcemia in COVID-19, this prospective cross-sectional study has been designed to evaluate the contributing factors involved in this disorder.
Materials and methods
Study design and characteristics of participants
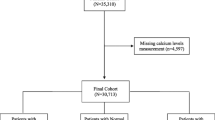
The current prospective cross-sectional study was conducted on hospitalized patients with COVID-19 in Booali education and therapeutic center, Qazvin Province, Iran, from January to April 2021. The inclusion criteria were age ≥ 18 years old, and confirmed COVID-19 by polymerase chain reaction (PCR) method. Patients with serum creatinine ≥ 2 mg/dl, known parathyroid or metabolic bone disease, advanced liver disease, or using anticonvulsants were excluded from the study. Information about demographic characteristics, comorbidities, and symptoms was collected and recorded in the questionnaire.
Serum total calcium and albumin were assessed on the first day of hospitalization, and serum samples were stored at − 80 °C. Serum total calcium, magnesium, and phosphate were assessed via colorimetric method using Selectra E Chemistry Analyzer (Hoogerheide, the Netherlands) and Delta Darman Kits.
The normal range of serum total calcium was 8.5–10.5 mg/dl with inter-assay and intra-assay 1.05% and 0.95%, respectively. Normal ranges of phosphate and magnesium were 2.5–4.5 mg/dl and 1.8–2.6 mg/dl, respectively. The parathormone (PTH) and 25 (OH)D assays were performed via Electrochemiluminescence (ECL) method using the Roche/Hitachi cobas® 6000 immunoassay system and Roche Kits with a normal range of 15–65 pg/ml and 30–70 ng/ml, respectively.
The participants were divided into three groups of normal calcium (serum total calcium ≥ 8.5 mg/dl), mild hypocalcemia (serum total calcium between 8 to 8.4 mg/dl), and moderate/severe hypocalcemia (serum total calcium lower than 8 mg/dl).
Vitamin D deficiency and insufficiency were defined as the serum 25 (OH)D lower than 20 ng/ml, and between 20–29.9 ng/ml, respectively.
Corrected calcium was calculated by the formula:
Based on laboratory criteria for diagnosis of hypoparathyroidism (low or low-normal PTH level in the presence of hypocalcemia [16], the term of functional hypoparathyroidism was used for PTH level in low/low normal range in the presence of hypocalcemia [11].
Statistical analysis
The Kolmogorov–Smirnov test was run to evaluate quantitative data distribution. The comparisons of quantitative data with normal distribution, abnormal distribution, and categorical data across the three groups were conducted using Analysis of variance (ANOVA), Kruskal–Wallis, and Chi-square tests, respectively. The logistic regression analysis was performed to investigate the independent associations of serum albumin, hypovitaminosis D, PTH status, and hypomagnesemia with hypocalcemia.
Results
Totally, 132 patients were recruited in the study. The serum creatinine of nine patients was > 2 mg/dl, and they were excluded from the study, and 123 patients remained for examining the cause of hypocalcemia. Demographic characteristics and disease severity indicators are given in Table 1.
In total, 65.9% and 60.2% of the patients had low total and corrected calcium, respectively. Frequencies of mild and moderate/severe hypocalcemia were 43.1% and 22.8%, respectively (Table 2).
The analysis regarding serum parameters involved in calcium metabolism is presented in Table 2.
Serum 25 (OH)D concentration was significantly higher in patients with normocalcemia compared to hypocalcemia groups (38.2 ± 16.2 ng/ml vs. 28.7 ± 19.5 ng/ml in mild and 25.3 ± 15.6 ng/ml in moderate/severe hypocalcemia, P = 0.01 and P = 0.004, respectively). This difference was also significant between mild low corrected calcium vs. normal corrected calcium groups (P = 0.003) (Table2).
Serum albumin was significantly different in calcium groups (4.1 ± 0.3 g/dl in normal serum total calcium vs. 3.9 ± 0.3 g/dl in mild hypocalcemia, and 3.5 ± 0.2 g/dl in moderate/severe hypocalcemia, P < 0.001). The comparisons of serum PTH, magnesium, and phosphate concentrations did not show any significant difference in serum total calcium or corrected calcium groups (Table 2).
The frequencies of abnormal values of the above serum parameters are represented in Fig. 1.
Status of biochemical and hormonal variables in different degrees of hypocalcemia. A1 Frequency of vitamin D deficiency and insufficiency in calcium groups. A2 Frequency of vitamin D deficiency and insufficiency in corrected calcium groups. B1 Frequency of functional hypoparathyroidism and secondary hyperparathyroidism in calcium groups. B2 Frequency of functional hypoparathyroidism and secondary hyperparathyroidism in corrected calcium groups. C1 Frequency of magnesium deficiency in calcium groups. C2 Frequency of magnesium deficiency in corrected calcium groups. D Frequency of serum albumin < 4 mg/dl in calcium groups
Regarding serum 25 (OH)D levels, 44.4% of the patients in moderate/severe and 37.7% in mild hypocalcemia groups had vitamin D deficiency. On the other hand, only 7.1% of patients in the normal serum total calcium group had vitamin D deficiency (P = 0.003). The frequency of vitamin D insufficiency was not significantly different across groups (Fig. 1). The frequency of vitamin D deficiency in moderate/severe hypocalcemia was nearly close to the prevalence of Iranian general population in the systematic review by Vatandost.et al. with prevalence of 56% [17].
In our study, only a minority of patients with hypocalcemia had appropriately high PTH (15.1% and 14.3% in mild and moderate/severe hypocalcemia, respectively), and the frequency of high PTH was not significantly different between normal and low serum total calcium groups (14.6% in the normal calcium group) (Fig. 1).
Functional hypoparathyroidism (low or low-normal PTH in the presence of hypocalcemia) was found in 45.3% and 28.6% of mild and moderate/severe hypocalcemia cases, respectively. When we considered corrected calcium, the frequency of functional hypoparathyroidism raised to 40.0% of moderate/severe low-corrected calcium (Fig. 1).
Hypomagnesemia was found with similar frequencies in calcium groups (10.7%, 9.4%, and 14.3% in moderate/severe, mild hypocalcemia, and normal calcium groups, in that order) (Fig. 1).
Serum albumin level < 4 g/dl was found in 92.9% of moderate/severe hypocalcemia vs 47.2% and 31.0% of mild hypocalcemia and normal calcium groups, respectively (P < 0.001) (Fig. 1).
The logistic regression analysis was performed to examine the independent roles of serum albumin, hypovitaminosis D, PTH status, and hypomagnesemia in the occurrence of hypocalcemia (Table 3).
After adjustment, vitamin D deficiency was associated with a 6.2 higher risk of hypocalcemia (CI 95% 2.0–19.5, P = 0.002). Furthermore, vitamin D insufficiency was associated with a 2.5 higher risk of hypocalcemia, but this association was not significant (P = 0.098).
Lower albumin levels were associated with low serum total calcium. For each 1 mg/dl decrease in the serum albumin from 4 mg/dl, the risk of low total calcium was increased 7.5 times (CI 95% 2.9–19.3, P < 0.001).
Other variables regarding hypomagnesemia, functional hypoparathyroidism or secondary hyperparathyroidism did not show any significant association with hypocalcemia.
Discussion
In the present study, about two-thirds of the patients with COVID-19 had hypocalcemia. Despite the higher frequency of relative hypoalbuminemia in the patients with hypocalcemia, the prevalence of low-corrected calcium was similarly high. Vitamin D deficiency was independently associated with about a six-times higher risk of hypocalcemia. We found a high prevalence of functional hypoparathyroidism in the patients with hypocalcemia. We did not find any role of serum magnesium disturbances in hypocalcemia.
There are some hypotheses as the causes of hypocalcemia in COVID-19 in special and critical illnesses in general. Hypoalbuminemia, vitamin D deficiency, decreased activity of 1α hydroxylase, functional hypoparathyroidism, PTH resistance, increased binding of calcium ions to unsaturated fatty acids, and virus-dependent mechanisms causing the influx of calcium into the cells are mentioned as the main reasons for hypocalcemia in COVID-19 [7, 11, 18, 19].
Vitamin D deficiency is common in critically ill patients with the reported prevalence of 40–70% [20]. Furthermore, prevalence of hypovitaminosis D is very high in patients with COVID-19. In the study by Hernández et al. about 82% of patients with COVID-19 had vitamin D deficiency. The mean of 25 (OH)D in this study was 13.8 ± 7.2 ng/ml [21]. In Pal et al.’s study, vitamin D deficiency was seen in 97% of patients with COVID-19, and the median of 25 (OH)D was as low as 9.1 ng/ml [15]. Despite this high prevalence of vitamin D deficiency in COVID-19, the association of this abnormality with hypocalcemia of COVID-19 is less investigated. In the prospective study by Osman et al., there was no association between serum 25 (OH)D and calcium levels [22].
Functional hypoparathyroidism and resistance to PTH action have been reported as contributing factors to hypocalcemia of critical illness [23]. In animal studies, high levels of interlukin1β induce up-regulation of calcium sensor receptors (CaSR) in the kidney and parathyroid cells. Other cytokines such as interlukin-6 and TNF-α also upregulate these receptors. These changes result in increasing sensitivity of CaSR to the serum total calcium and higher threshold of parathyroid cells for PTH secretion [24, 25]. Furthermore, magnesium deficiency can impair PTH secretion response to hypocalcemia and PTH resistance [26].
In Lind et al.’s study in septic patients with hypocalcemia, PTH level was increased in more than seventy percent of hypocalcemic patients. In a minority of the patients, PTH level remained in normal range. These patients with low PTH concentrations had significantly higher CRP and inflammatory cytokines [27].
In Hu et al.’s study conducted on patients admitted to medical intensive care unit, about ninety percent of patients had low ionized calcium. Secondary hyperparathyroidism occurred in 56% of hypocalcemic patients [28].
Surprisingly, appropriately high PTH was found in only about one-seventh of our patients with hypocalcemia. In about 40% of the patients with moderate/severe low-corrected calcium, the PTH level was low or in the lower half of the normal range. The reason of this difference between COVID-19 and above mentioned critical illnesses is not clear, but may be one of the clues of highly prevalent hypocalcemia even in non-severe COVID-19.
The serum magnesium level did not have a significant difference in hypocalcemia and normocalcemia; however, serum magnesium level is not a good indicator of intracellular magnesium [29]. Thus we cannot reject total body magnesium deficiency as a contributing factor in hypocalcemia.
Our study had some limitations. The study design was cross-sectional, so causality could not be confirmed. Another limitation was the lack of assessment of ionized calcium. We calculated corrected calcium, but this parameter does not have a good sensitivity in detecting hypocalcemia in critical illnesses [30]. The third limitation was the inability to evaluate some hypotheses about hypocalcemia in COVID-19 such as increased binding of Ca + + to unsaturated fatty acids and virus-dependent increased influx of Ca + + into the cells.
In conclusion, the results of our study revealed the significant roles of vitamin D deficiency and the high frequency of functional hypoparathyroidism in the hypocalcemia of COVID-19.
References
Mohammadi F, Badri M, Safari S, Hemmat N et al (2021) A case report of rhino-facial mucormycosis in a non-diabetic patient with COVID-19: a systematic review of literature and current update. BMC Infect Dis 21:906. https://doi.org/10.1186/s12879-021-06625-3
Rubino F, Amiel SA, Zimmet P, Alberti G, Bornstein S et al (2020) New-onset diabetes in Covid-19. N Engl J Med 383:789–790
Yousaf Z, Al-Shokri SD, Al-Soub H, Mohamed MF (2020) COVID-19-associated SIADH: a clue in the times of pandemic! Am J Physiol-Endocrinol Metab 318:E882–E885
Marazuela M, Giustina A, Puig-Domingo M (2020) Endocrine and metabolic aspects of the COVID-19 pandemic. Rev Endocr Metab Disord 21:495–507
Di Filippo L, Formenti AM, Rovere-Querini P, Carlucci M, Conte C et al (2020) Hypocalcemia is highly prevalent and predicts hospitalization in patients with COVID-19. Endocrine 68:475–478
Liu J, Han P, Wu J, Gong J, Tian D (2020) Prevalence and predictive value of hypocalcemia in severe COVID-19 patients. J Infect Public Health 13:1224–1228
Sun J-K, Zhang W-H, Zou L, Liu Y, Li J-J et al (2020) Serum calcium as a biomarker of clinical severity and prognosis in patients with coronavirus disease 2019. Aging (Albany NY) 12:11287
Bennouar S, Cherif AB, Kessira A, Bennouar D-E, Abdi S (2020) Vitamin D deficiency and low serum calcium as predictors of poor prognosis in patients with severe COVID-19. J Am Coll Nutr 40:104–110
Zaloga GP, Chernow B (1986) Hypocalcemia in critical illness. JAMA 256:1924–1929
Desai TK, Carlson RW, Geheb MA (1988) Prevalence and clinical implications of hypocalcemia in acutely III patients in a medical intensive care setting. Am J Med 84:209–214
Kelly A, Levine MA (2013) Hypocalcemia in the critically ill patient. J Intensive Care Med 28:166–177
Lee P (2011) Vitamin D metabolism and deficiency in critical illness. Best Pract Res Clin Endocrinol Metab 25:769–781
Quraishi SA, Camargo CA Jr (2012) Vitamin D in acute stress and critical illness. Curr Opin Clin Nutr Metab Care 15:625
di Filippo L, Formenti AM, Doga M, Frara S, Rovere-Querini P et al (2021) Hypocalcemia is a distinctive biochemical feature of hospitalized COVID-19 patients. Endocrine 71:9–13
Pal R, Ram S, Zohmangaihi D, Biswas I, Suri V et al (2020) High prevalence of hypocalcemia in non-severe COVID-19 patients: a retrospective case-control study. Front Med 7:1057
Bilezikian JP, Khan A, Potts JT Jr, Brandi ML, Clarke BL et al (2011) Hypoparathyroidism in the adult: Epidemiology, diagnosis, pathophysiology, target-organ involvement, treatment, and challenges for future research. J Bone Miner Res 26:2317–2337
Vatandost S, Jahani M, Afshari A, Amiri MR, Heidarimoghadam R, Mohammadi Y (2018) Prevalence of vitamin D deficiency in Iran: a systematic review and meta-analysis. Nutr Health 24:269–278
di Filippo L, Doga M, Frara S, Giustina A (2021) Hypocalcemia in COVID-19: prevalence, clinical significance and therapeutic implications. Rev Endocr Metab Disord 23:299–308
Zhou Y, Frey TK, Yang JJ (2009) Viral calciomics: interplays between Ca2+ and virus. Cell Calcium 46:1–17
Amrein K, Papinutti A, Mathew E, Vila G, Parekh D (2018) Vitamin D and critical illness: what endocrinology can learn from intensive care and vice versa. Endocr Connect 7:R304–R315
Hernández JL, Nan D, Fernandez-Ayala M, García-Unzueta M, Hernández-Hernández MA et al (2021) Vitamin D status in hospitalized patients with SARS-CoV-2 infection. J Clin Endocrinol Metab 106:e1343–e1353
Osman W, Al Fahdi F, Al Salmi I, Al Khalili H, Gokhale A, Khamis F (2021) Serum calcium and vitamin D levels: correlation with severity of COVID-19 in hospitalized patients in Royal Hospital. Oman Int J Infect Dis 107:153–163
Nair P, Lee P, Reynolds C, Nguyen ND, Myburgh J et al (2013) Significant perturbation of vitamin D–parathyroid–calcium axis and adverse clinical outcomes in critically ill patients. Intensive Care Med 39:267–274
Canaff L, Hendy GN (2005) Calcium-sensing receptor gene transcription Is up-regulated by the proinflammatory cytokine, interleukin-1β: role of the NF-κB pathway and κB elements. J Biol Chem 280:14177–14188
Canaff L, Zhou X, Hendy GN (2008) The proinflammatory cytokine, interleukin-6, up-regulates calcium-sensing receptor gene transcription via Stat1/3 and Sp1/3. J Biol Chem 283:13586–13600
Tong GM, Rude RK (2005) Magnesium deficiency in critical illness. J Intensive Care Med 20:3–17
Lind L, Carlstedt F, Rastad J, Stiernström H, Stridsberg M et al (2000) Hypocalcemia and parathyroid hormone secretion in critically ill patients. Crit Care Med 28:93–99
Hu J, Luo Z, Zhao X, Chen Q, Chen Z et al (2013) Changes in the calcium-parathyroid hormone-vitamin d axis and prognosis for critically ill patients: a prospective observational study. PLoS ONE 8:e75441
Swaminathan R (2003) Magnesium metabolism and its disorders. Clin Biochem Rev 24:47
Dickerson RN, Alexander KH, Minard G, Croce MA, Brown RO (2004) Accuracy of methods to estimate ionized and “corrected” serum calcium concentrations in critically ill multiple trauma patients receiving specialized nutrition support. J Parenter Enter Nutr 28:133–141
Funding
This work was supported by the Metabolic Diseases Research Center, Research Institute for Prevention of Non-Communicable Diseases, Qazvin University of Medical Sciences, Qazvin, Iran under the contract no. IR.QUMS.Rec.1399.565
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declared no potential conflicts of interest with respect to the research, authorship and/or publication of this article.
Ethical approval
The research protocols used in this research were approved by the ethics committee and the research council of Qazvin University of Medical Sciences, Qazvin, Iran (The ethical cod: IR.QUMS.Rec.1399.565).
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
About this article
Cite this article
Hashemipour, S., Kiani, S., Shahsavari, P. et al. Hypocalcemia in hospitalized patients with COVID-19: roles of hypovitaminosis D and functional hypoparathyroidism. J Bone Miner Metab 40, 663–669 (2022). https://doi.org/10.1007/s00774-022-01330-w
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00774-022-01330-w