Abstract
Introduction
Periostin, as an emerging biomarker, is involved in multiple steps in bone metabolism. This study aimed to investigate the correlation between periostin levels and bone mineral density as well as bone turnover markers in postmenopausal women with type 2 diabetes (T2DM).
Materials and methods
This study was a cross-sectional study that included 164 postmenopausal women with T2DM as study subjects and 32 age-matched nondiabetic postmenopausal women with normal bone mineral density (BMD) as healthy control subjects. A total of 164 subjects with T2DM were then divided into three groups according to BMD: the normal BMD group (n = 29), the osteopenia group (n = 70), and the osteoporosis group (n = 65). The clinical data of all subjects along with the relevant biochemical parameter data were collected. Plasma periostin was detected using an enzyme-linked immunosorbent assay (ELISA).
Results
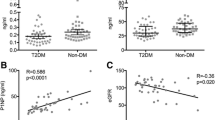
Plasma periostin levels were significantly increased in T2DM patients with normal BMD compared with healthy controls (p < 0.05). In the diabetic group, plasma periostin levels were significantly elevated with decreased BMD, were positively correlated with osteocalcin levels (r = 0.162, p = 0.039) and were inversely associated with femoral neck BMD (r = − 0.308, p < 0.001) and total femur BMD (r = − 0.295, p < 0.001). In the case of chronic complications, periostin levels were slightly increased in individuals with complications of diabetic retinopathy, diabetic nephropathy and fracture (p > 0.05).
Conclusions
The current study demonstrated that plasma periostin levels were significantly associated with BMD in patients with T2DM, and periostin might act as a novel biochemical marker of osteoporosis in postmenopausal women with type 2 diabetes.
Similar content being viewed by others
References
Waterloo S, Nguyen T, Ahmed LA, Center JR, Morseth B, Nguyen ND, Eisman JA, Søgaard AJ, Emaus N (2012) Important risk factors and attributable risk of vertebral fractures in the population-based Tromsø study. BMC Musculoskelet Disord 13:163
Schwartz AV, Vittinghoff E, Bauer DC, Hillier TA, Strotmeyer ES, Ensrud KE, Donaldson MG, Cauley JA, Harris TB, Koster A (2011) Association of BMD and FRAX score with risk of fracture in older adults with type 2 diabetes. JAMA 305:2184–2192
Nilsson AG, Sundh D, Johansson L, Nilsson M, Mellström D, Rudäng R, Zoulakis M, Wallander M, Darelid A, Lorentzon M (2017) Type 2 diabetes mellitus is associated with better bone microarchitecture but lower bone material strength and poorer physical function in elderly women: a population-based study. J Bone Miner Res 32:1062–1071
Patsch JM, Burghardt AJ, Yap SP, Baum T, Schwartz AV, Joseph GB, Link TM (2013) Increased cortical porosity in type 2 diabetic postmenopausal women with fragility fractures. J Bone Miner Res 28:313–324
Bala Y, Bui QM, Wang XF, Iuliano S, Wang Q, Ghasem-Zadeh A, Rozental TD, Bouxsein ML, Zebaze RM, Seeman E (2015) Trabecular and cortical microstructure and fragility of the distal radius in women. J Bone Miner Res 30:621–629
Yu E, Putman MS, Derrico N, Abrishamanian-Garcia G, Finkelstein JS, Bouxsein ML (2015) Defects in cortical microarchitecture among African–American women with type 2 diabetes. Osteoporos Int 26:673–679
Heilmeier U, Cheng K, Pasco C, Parrish R, Nirody J, Patsch J, Zhang C, Joseph G, Burghardt A, Schwartz A (2016) Cortical bone laminar analysis reveals increased midcortical and periosteal porosity in type 2 diabetic postmenopausal women with history of fragility fractures compared to fracture-free diabetics. Osteoporos Int 27:2791–2802
Holzer G, Von Skrbensky G, Holzer LA, Pichl W (2009) Hip fractures and the contribution of cortical versus trabecular bone to femoral neck strength. J Bone Miner Res 24:468–474
Li G, Jin R, Norris RA, Zhang L, Yu S, Wu F, Markwald RR, Nanda A, Conway SJ, Smyth SS (2010) Periostin mediates vascular smooth muscle cell migration through the integrins ανβ3 and ανβ5 and focal adhesion kinase (FAK) pathway. Atherosclerosis 208:358–365
Kudo A (2011) Periostin in fibrillogenesis for tissue regeneration: periostin actions inside and outside the cell. Cell Mol Life Sci 68:3201
Bonnet N, Rousseau J, Garnero P, Ferrari S (2011) Periostin: a novel serum marker of cortical bone formation. J Bone Miner Res:SU0113
Fortunati D, Reppe S, Fjeldheim Å-K, Nielsen M, Gautvik VT, Gautvik KM (2010) Periostin is a collagen associated bone matrix protein regulated by parathyroid hormone. Matrix Biol 29:594–601
Bonnet N, Standley KN, Bianchi EN, Stadelmann V, Foti M, Conway SJ, Ferrari SL (2009) The matricellular protein periostin is required for sost inhibition and the anabolic response to mechanical loading and physical activity. J Biol Chem 284:35939–35950
Nakazawa T, Nakajima A, Seki N, Okawa A, Kato M, Moriya H, Amizuka N, Einhorn TA, Yamazaki M (2004) Gene expression of periostin in the early stage of fracture healing detected by cDNA microarray analysis. J Orthop Res 22:520–525
Bonnet N, Gineyts E, Ammann P, Conway SJ, Garnero P, Ferrari S (2013) Periostin deficiency increases bone damage and impairs injury response to fatigue loading in adult mice. PLoS ONE 8:e78347
Walsh JS, Gossiel F, Scott JR, Paggiosi MA, Eastell R (2017) Effect of age and gender on serum periostin: relationship to cortical measures, bone turnover and hormones. Bone 99:8–13
Gerbaix M, Vico L, Ferrari SL, Bonnet N (2015) Periostin expression contributes to cortical bone loss during unloading. Bone 71:94–100
Contié S, Voorzanger-Rousselot N, Litvin J, Bonnet N, Ferrari S, Clézardin P, Garnero P (2010) Development of a new ELISA for serum periostin: evaluation of growth-related changes and bisphosphonate treatment in mice. Calcif Tissue Int 87:341–350
Anastasilakis A, Polyzos S, Makras P, Savvides M, Sakellariou G, Gkiomisi A, Papatheodorou A, Terpos E (2014) Circulating periostin levels do not differ between postmenopausal women with normal and low bone mass and are not affected by zoledronic acid treatment. Horm Metab Res 46:145–149
Merle B, Garnero P (2012) The multiple facets of periostin in bone metabolism. Osteoporos Int 23:1199–1212
Xiao S-M, Gao Y, Cheung C-L, Bow C, Lau K-S, Sham P, Tan K, Kung A (2012) Association of CDX1 binding site of periostin gene with bone mineral density and vertebral fracture risk. Osteoporos Int 23:1877–1887
Kim B-J, Rhee Y, Kim CH, Baek KH, Min Y-K, Kim D-Y, Ahn SH, Kim H, Lee SH, Lee S-Y (2015) Plasma periostin associates significantly with non-vertebral but not vertebral fractures in postmenopausal women: clinical evidence for the different effects of periostin depending on the skeletal site. Bone 81:435–441
Polyzos SA, Kountouras J, Anastasilakis AD, Papatheodorou A, Kokkoris P, Terpos E (2017) Circulating periostin in patients with nonalcoholic fatty liver disease. Endocrine 56:438–441
Yang Z, Zhang H, Niu Y, Zhang W, Zhu L, Li X, Lu S, Fan J, Li X, Ning G (2016) Circulating periostin in relation to insulin resistance and nonalcoholic fatty liver disease among overweight and obese subjects. Sci Rep 6:1–6
Luo Y, Qu H, Wang H, Wei H, Wu J, Duan Y, Liu D, Deng H (2016) Plasma periostin levels are increased in Chinese subjects with obesity and type 2 diabetes and are positively correlated with glucose and lipid parameters. Mediat Inflamm 2016:6423637
Ding Y, Ge Q, Qu H, Feng Z, Long J, Wei Q, Zhou Q, Wu R, Yao L, Deng H (2018) Increased serum periostin concentrations are associated with the presence of diabetic retinopathy in patients with type 2 diabetes mellitus. J Endocrinol Invest 41:937–945
Yoshida S, Ishikawa K, Asato R, Arima M, Sassa Y, Yoshida A, Yoshikawa H, Narukawa K, Obika S, Ono J (2011) Increased expression of periostin in vitreous and fibrovascular membranes obtained from patients with proliferative diabetic retinopathy. Invest Ophthalmol Vis Sci 52:5670–5678
El-Dawla NMQ, Sallam A-AM, El-Hefnawy MH, El-Mesallamy HO (2019) E-cadherin and periostin in early detection and progression of diabetic nephropathy: epithelial-to-mesenchymal transition. Clin Exp Nephrol 23:1050–1057
Satirapoj B, Tassanasorn S, Charoenpitakchai M, Supasyndh O (2015) Periostin as a tissue and urinary biomarker of renal injury in type 2 diabetes mellitus. PLoS ONE 10:e0124055
Ghafar MTA, Shalaby KH, Okda HI, Soliman NA, Keshk WA (2020) Assessment of two novel renal tubular proteins in type 2 diabetic patients with nephropathy. J Investig Med 68:748–755
Singh S, Kumar D, Lal AK (2015) Serum osteocalcin as a diagnostic biomarker for primary osteoporosis in women. J Clin Diagn Res 9:RC04
Rousseau J, Sornay-Rendu E, Bertholon C, Chapurlat R, Garnero P (2014) Serum periostin is associated with fracture risk in postmenopausal women: a 7-year prospective analysis of the OFELY study. J Clin Endocrinol Metabol 99:2533–2539
Mehta T, Bůžková P, Sarnak MJ, Chonchol M, Cauley JA, Wallace E, Fink HA, Robbins J, Jalal D (2015) Serum urate levels and the risk of hip fractures: data from the Cardiovascular Health Study. Metabolism 64:438–446
Wauquier F, Leotoing L, Coxam V, Guicheux J, Wittrant Y (2009) Oxidative stress in bone remodelling and disease. Trends Mol Med 15:468–477
Sakellariou GT, Anastasilakis AD, Bisbinas I, Oikonomou D, Gerou S, Polyzos SA, Sayegh FE (2014) Circulating periostin levels in patients with AS: association with clinical and radiographic variables, inflammatory markers and molecules involved in bone formation. Rheumatology 54:908–914
Wang F, Yang C, Song Y, Jiang Y, Ding Z (2012) Periostin gene polymorphisms, protein levels and risk of incident coronary artery disease. Mol Biol Rep 39:359–367
Acknowledgements
We are deeply grateful to all the study participants. Funding was received from National Natural Science Foundation of China (31871281) and Scientific Research Foundation for Advanced Talents of Shanghai University of Traditional Chinese Medicine to Dr. Qingzhong Wang. This study was also supported by Shanxi Province Higher Education Innovation Project (2019l0701) to Dr. Junyan li.
Author information
Authors and Affiliations
Contributions
WQ and JiL designed the project. Material preparation and data collection and analysis were performed by JuL, XN, and QS. MJ, RZ and YS measured the periostin level. The first draft of the manuscript was written by JuL. QW and JL checked and edited the manuscripts. All authors read and approved the final version of the manuscript.
Corresponding authors
Ethics declarations
Conflict of interest
Junyan Li, Xiaohong Niu, Qinqin Si, Qi Song, Miaomiao Jin, Ruijun Zhou, Jianbo Li and Qingzhong Wang declare that they have no conflicts of interest.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
About this article
Cite this article
Li, J., Niu, X., Si, Q. et al. Plasma periostin as a biomarker of osteoporosis in postmenopausal women with type 2 diabetes. J Bone Miner Metab 39, 631–638 (2021). https://doi.org/10.1007/s00774-020-01200-3
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00774-020-01200-3