Abstract
Introduction
Denosumab is a humanized IgG2 monoclonal antibody that was approved for the treatment of osteoporosis in Japan in 2013. This study aimed to investigate the long-term safety and effectiveness of denosumab in Japanese patients with osteoporosis in daily clinical practice.
Materials and methods
This 3-year, prospective, observational, post-marketing study included patients who initiated treatment with denosumab (60 mg/6 months) for osteoporosis. Data were assessed at baseline, 3, 6, 12, 24 and 36 months. Key endpoints were adverse events (AEs), adverse drug reactions (ADRs), occurrence of osteoporotic fractures, bone mineral density (BMD), and bone turnover markers. Multivariate analyses were conducted to identify predictors of hypocalcaemia and percent change in BMD.
Results
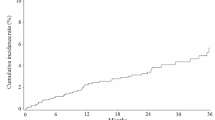
Overall, 3534 patients were assessed (mean 75.7 years; 89.8% women). In total, 298 patients (8.4%) developed ADRs; the most common was hypocalcaemia (3.9%). Hypocalcaemia risk was significantly increased in patients with creatinine clearance < 30 mL/min, no prior use of bisphosphonates, prior use of calcium and vitamin D preparations, baseline serum calcium < 8.5 mg/dL, and no concomitant use of calcium or vitamin D preparations. Six patients had adjudicated osteonecrosis of the jaw. Lumbar spine BMD increased significantly from baseline (mean percent change: 11.4% at 36 months). All bone turnover markers decreased significantly from baseline. Over 3 years, 3.3% of patients developed a new osteoporotic fracture.
Conclusions
This study confirmed the long-term safety and effectiveness of denosumab in Japanese patients with osteoporosis in daily clinical practice. No new safety signals were identified.


Similar content being viewed by others
References
Black DM, Rosen CJ (2016) Clinical practice. Postmenopausal Osteoporos N Engl J Med 374:254–262
Orimo H, Nakamura T, Hosoi T, Iki M, Uenishi K, Endo N, Ohta H, Shiraki M, Sugimoto T, Suzuki T, Soen S, Nishizawa Y, Hagino H, Fukunaga M, Fujiwara S (2012) Japanese 2011 guidelines for prevention and treatment of osteoporosis—executive summary. Arch Osteoporos 7:3–20
Rachner TD, Khosla S, Hofbauer LC (2011) Osteoporosis: now and the future. Lancet 377:1276–1287
Johnell O, Kanis JA (2006) An estimate of the worldwide prevalence and disability associated with osteoporotic fractures. Osteoporos Int 17:1726–1733
Kitamura S, Hasegawa Y, Suzuki S, Sasaki R, Iwata H, Wingstrand H, Thorngren KG (1998) Functional outcome after hip fracture in Japan. Clin Orthop Relat Res 348:29–36
Iki M (2012) Epidemiology of osteoporosis in Japan (in Japanese). Clin Calcium 22:797–803
International Osteoporosis Foundation (2009) The Asian Audit: epidemiology, costs and burden of osteoporosis in Asia 2009. https://www.iofbonehealth.org/sites/default/files/PDFs/Audit%20Asia/Asian_regional_audit_2009.pdf. Accessed 3 Feb 2020
Lacey DL, Boyle WJ, Simonet WS, Kostenuik PJ, Dougall WC, Sullivan JK, San Martin J, Dansey R (2012) Bench to bedside: elucidation of the OPG-RANK-RANKL pathway and the development of denosumab. Nat Rev Drug Discov 11:401–419
Bekker PJ, Holloway DL, Rasmussen AS, Murphy R, Martin SW, Leese PT, Holmes GB, Dunstan CR, DePaoli AM (2004) A single-dose placebo-controlled study of AMG 162, a fully human monoclonal antibody to RANKL, in postmenopausal women. J Bone Miner Res 19:1059–1066
McClung MR (2006) Inhibition of RANKL as a treatment for osteoporosis: preclinical and early clinical studies. Curr Osteoporos Rep 4:28–33
Cummings SR, San Martin J, McClung MR, Siris ES, Eastell R, Reid IR, Delmas P, Zoog HB, Austin M, Wang A, Kutilek S, Adami S, Zanchetta J, Libanati C, Siddhanti S, Christiansen C (2009) Denosumab for prevention of fractures in postmenopausal women with osteoporosis. N Engl J Med 361:756–765
Nakamura T, Matsumoto T, Sugimoto T, Hosoi T, Miki T, Gorai I, Yoshikawa H, Tanaka Y, Tanaka S, Sone T, Nakano T, Ito M, Matsui S, Yoneda T, Takami H, Watanabe K, Osakabe T, Shiraki M, Fukunaga M (2014) Clinical trials express: fracture risk reduction with denosumab in Japanese postmenopausal women and men with osteoporosis: denosumab fracture intervention randomized placebo controlled trial (DIRECT). J Clin Endocrinol Metab 99:2599–2607
Bone HG, Wagman RB, Brandi ML, Brown JP, Chapurlat R et al (2017) 10 years of denosumab treatment in postmenopausal women with osteoporosis: results from the phase 3 randomised FREEDOM trial and open-label extension. Lancet Diabetes Endocrinol 5:513–523
Sugimoto T, Matsumoto T, Hosoi T, Miki T, Gorai I et al (2015) Three-year denosumab treatment in postmenopausal Japanese women and men with osteoporosis: results from a 1-year open-label extension of the Denosumab Fracture Intervention Randomized Placebo Controlled Trial (DIRECT). Osteoporos Int 26:765–774
Tanaka S, Mizutani H, Tsuruya E, Mochikuzi Y, Kuge K, Okubo N (2018) Safety and efficacy of long-term denosumab treatment in Japanese patients with osteoporosis in daily clinical practice: an interim analysis of a post-marketing surveillance study. Ther Res 39:453–465
Ruggiero SL, Dodson TB, Fantasia J, Goodday R, Aghaloo T, Mehrotra B, O’Ryan F (2014) American association of oral and maxillofacial surgeons position paper on medication-related osteonecrosis of the jaw—2014 update. J Oral Maxillofac Surg 72:1938–1956
Clopper CJ, Pearson ES (1934) The use of confidence or fiducial limits illustrated in the case of the binomial. Biometrika 26:404–413
Price D, Bateman ED, Chisholm A, Papadopoulos NG, Bosnic-Anticevich S, Pizzichini E, Hillyer EV, Buist AS (2014) Complementing the randomized controlled trial evidence base. Evolution not revolution. Ann Am Thorac Soc 11:S92–S98
McClung MR, Boonen S, Torring O, Roux C, Rizzoli R, Bone HG, Benhamou CL, Lems WF, Minisola S, Halse J, Hoeck HC, Eastell R, Wang A, Siddhanti S, Cummings SR (2012) Effect of denosumab treatment on the risk of fractures in subgroups of women with postmenopausal osteoporosis. J Bone Miner Res 27:211–218
Iidaka T, Muraki S, Akune T, Oka H, Kodama R, Tanaka S, Kawaguchi H, Nakamura K, Yoshimura N (2016) Prevalence of radiographic hip osteoarthritis and its association with hip pain in Japanese men and women: the ROAD study. Osteoarthr Cartil 24:117–123
Kamatari M, Koto S, Ozawa N, Urao C, Suzuki Y, Akasaka E, Yanagimoto K, Sakota K (2007) Factors affecting long-term compliance of osteoporotic patients with bisphosphonate treatment and QOL assessment in actual practice: alendronate and risedronate. J Bone Miner Metab 25:302–309
Ruggiero SL, Dodson TB, Assael LA, Landesberg R, Marx RE, Mehrotra B (2009) American association of oral and maxillofacial surgeons position paper on bisphosphonate-related osteonecrosis of the jaws—2009 update. J Oral Maxillofac Surg 67:2–12
Acknowledgements
We are deeply grateful to the investigators and participants of the survey. This study was sponsored by Daiichi Sankyo Co., Ltd. (Tokyo, Japan). In addition, the authors would like to thank Go Kuratomi, PhD, and Catherine Rees, of Springer Healthcare Communications, who wrote the outline and the subsequent drafts of this manuscript, respectively, and Sarah Greig, PhD, of Springer Healthcare Communications who assisted with manuscript revisions post-submission. This medical writing assistance was funded by Daiichi Sankyo Co., Ltd. This study was sponsored by Daiichi Sankyo Co., Ltd. (Tokyo, Japan).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
S. Tanaka received consulting fees, speaking fees and/or honoraria from Daiichi Sankyo Co., Ltd. H. Mizutani, E. Tsuruya, R. Fukuda, K. Kuge and N. Okubo are employees of Daiichi Sankyo Co., Ltd.
Ethical approval
This study was conducted in accordance with the Japanese GPSP guidelines. Under these guidelines, the requirement for written informed consent is waived because treatment is in accordance with clinical practice.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
Below is the link to the electronic supplementary material.
About this article
Cite this article
Tanaka, S., Mizutani, H., Tsuruya, E. et al. Long-term safety and effectiveness of denosumab in Japanese patients with osteoporosis: 3-year post-marketing surveillance study. J Bone Miner Metab 39, 463–473 (2021). https://doi.org/10.1007/s00774-020-01180-4
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00774-020-01180-4