Abstract
Introduction
Bisphosphonates are the standard treatment for glucocorticoid-induced osteoporosis (GIOP) with teriparatide being another option. While daily teriparatide has been shown to be effective in increasing bone mineral density (BMD), the efficacy of once-weekly teriparatide (56.5 µg) has not yet been evaluated. The TOWER-GO study, a 72-week, multicenter, open-label, randomized controlled trial, was conducted in patients with GIOP to compare the effects of once-weekly teriparatide and once-weekly alendronate 35 mg on BMD.
Materials and methods
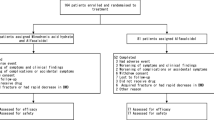
Patients (N = 180) with GIOP for whom drug treatment was indicated according to the 2004 guidelines in Japan were randomized to receive once-weekly teriparatide (n = 89) or once-weekly alendronate (n = 91). The primary endpoint was the non-inferiority of percentage change in lumbar spine BMD at final follow-up. The secondary endpoints were the percentage change in BMD from baseline, incidence of bone fractures, and changes in bone turnover markers.
Results
While the non-inferiority of teriparatide to alendronate was not confirmed, BMD increased significantly from baseline with teriparatide and alendronate by 5.09% and 4.04%, respectively (both p < 0.05), at 72 weeks. The incidence of vertebral and non-vertebral fractures was similar in both groups. Bone formation markers increased in the teriparatide group and decreased in the alendronate group.
Conclusions
The non-inferiority of once-weekly teriparatide versus once-weekly alendronate in BMD change at 72 weeks was not shown, but the increase in bone formation markers over time and the increase of BMD in GIOP patients treated with once-weekly teriparatide were confirmed.




Similar content being viewed by others
References
NIH-National Institutes of Health (2000) Osteoporosis prevention, diagnosis, and therapy. NIH Consens Statement 17:1–45
Akkawi I, Zmerly H (2018) Osteoporosis: current concepts. Joints 6:122–127
Weinstein RS (2011) Clinical practice. Glucocorticoid-induced bone disease. N Engl J Med 365:62–70
Nawata H, Soen S, Takayanagi R, Tanaka I, Takaoka K, Fukunaga M, Matsumoto T, Suzuki Y, Tanaka H, Fujiwara S, Miki T, Sagawa A, Nishizawa Y, Seino Y, Guidelines on the management and treatment of glucocorticoid-induced osteoporosis of the Japanese Society for Bone and Mineral Research (2005) Guidelines on the management and treatment of glucocorticoid-induced osteoporosis of the Japanese Society for Bone and Mineral Research (2004). J Bone Miner Metab 23:105–109
Suzuki Y, Nawata H, Soen S, Fujiwara S, Nakayama H, Tanaka I, Ozono K, Sagawa A, Takayanagi R, Tanaka H, Miki T, Masunari N, Tanaka Y (2014) Guidelines on the management and treatment of glucocorticoid-induced osteoporosis of the Japanese Society for Bone and Mineral Research: 2014 update. J Bone Miner Metab 32:337–350
Buckley L, Guyatt G, Fink HA, Cannon M, Grossman J et al (2017) 2017 American College of Rheumatology Guideline for the prevention and treatment of glucocorticoid-induced osteoporosis. Arthritis Rheumatol 69:1521–1537
Saag KG, Shane E, Boonen S, Marín F, Donley DW, Taylor KA, Dalsky GP, Marcus R (2007) Teriparatide or alendronate in glucocorticoid-induced osteoporosis. N Engl J Med 357:2028–2039
Nakamura T, Sugimoto T, Nakano T, Kishimoto H, Ito M, Fukunaga M, Hagino H, Sone T, Yoshikawa H, Nishizawa Y, Fujita T, Shiraki M (2012) Randomized Teriparatide [human parathyroid hormone (PTH) 1–34] Once-Weekly Efficacy Research (TOWER) trial for examining the reduction in new vertebral fractures in subjects with primary osteoporosis and high fracture risk. J Clin Endocrinol Metab 97:3097–3106
Uchida S, Taniguchi T, Shimizu T, Kakikawa T, Okuyama K, Okaniwa M, Arizono H, Nagata K, Santora AC, Shiraki M, Fukunaga M, Tomomitsu T, Ohashi Y, Nakamura T (2005) Therapeutic effects of alendronate 35 mg once weekly and 5 mg once daily in Japanese patients with osteoporosis: a double-blind, randomized study. J Bone Miner Metab 23:382–388
Adachi JD, Olszynski WP, Hanley DA, Hodsman AB, Kendler DL, Siminoski KG, Brown J, Cowden EA, Goltzman D, Ioammidis G, Josse RG, Ste-Marie LG, Tenenhouse AM, Davison KS, Blocka KL, Pollock AP, Sibley J (2000) Management of corticosteroid-induced osteoporosis. Semin Arthritis Rheum 29:228–251
American College of Rheumatology Ad Hoc Committee on Glucocorticoid-Induced Osteoporosis (2001) Recommendations for the prevention and treatment of glucocorticoid-induced osteoporosis. Update Arthritis Rheum 44:1496–1503
Brown JP, Josse RG (2002) Clinical practice guidelines for the diagnosis and management of osteoporosis in Canada. Can Med Assoc J 167:1S-34S
Bone and Tooth Society, National Osteoporosis Society, Royal College of Physicians (2002) Glucocorticoid-induced osteoporosis: guidelines for prevention and treatment. Royal College of Physicians, London
Van Staa TP, Leufkens HG, Cooper C (2002) The epidemiology of corticosteroid-induced osteoporosis: a meta-analysis. Osteoporos Int 13:777–787
Genant HK, Wu CY, van Kuijk C, Nevitt MC (1993) Vertebral fracture assessment using a semiquantitative technique. J Bone Miner Res 8:1137–1148
Genant HK, Jerges M, Palermo L, Nevitt M, Valentin RS, Black D, Cummings SR (1996) Comparison of semiquantitative visual and quantitative morphometric assessment of prevalent and incident vertebral fractures in osteoporosis. The study of osteoporotic fractures research group. J Bone Miner Res 11:984–996
Kishimoto H, Fukunaga M, Kushida K, Shiraki M, Itabashi A, Nawata H, Nakamura T, Ohta H, Takaoka K, Ohashi Y, Phase III Research Group (2006) Efficacy and tolerability of once-weekly administration of 17.5 mg risedronate in Japanese patients with involutional osteoporosis: a comparison with 2.5-mg once-daily dosage regimen. J Bone Miner Metab 24:405–413
Saag KG, Emkey R, Schnitzer TJ, Brown JP, Hawkins F, Goemaere S, Thamsborg G, Liberman UA, Delmas PD, Malice MP, Czachur M, Daifotis AG (1998) Alendronate for the prevention and treatment of glucocorticoid-induced osteoporosis. Glucocorticoid-Induced Osteoporosis Intervention Study Group. N Engl J Med 339:292–299
Stoch SA, Saag KG, Greenwald M, Sebba AI, Cohen S, Verbruggen N, Giezek H, West J, Schnitzer TJ (2009) Once-weekly oral alendronate 70 mg in patients with glucocorticoid-induced bone loss: a 12-month randomized, placebo-controlled clinical trial. J Rheumatol 36:1705–1714
Briot K, Roux C (2015) Glucocorticoid-induced osteoporosis RMD Open 1:e000014
Taylor AD, Saag KG (2019) Anabolics in the management of glucocorticoid-induced osteoporosis: an evidence-based review of long-term safety, efficacy and place in therapy. Core Evid 14:41–50
Okada Y, Nawata M, Nakayamada S, Saito K, Tanaka Y (2008) Alendronate protects premenopausal women from bone loss and fracture associated with high-dose glucocorticoid therapy. J Rheumatol 35:2249–2254
van Staa TP, Leufkens HG, Abenhaim L, Zhang B, Cooper C (2000) Use of oral corticosteroids and risk of fractures. J Bone Miner Res 15:993–1000
Takeuchi Y (2019) How different is once-weekly teriparatide from the daily once or the same? Osteoporos Sarcopenia 5:27–28
McClung MR, San Martin J, Miller PD, Civitelli R, Bandeira F, Omizo M, Donley DW, Dalsky GP, Eriksen EF (2005) Opposite bone remodeling effects of teriparatide and alendronate in increasing bone mass. Arch Intern Med 165:1762–1768
Ifuku E, Yoshimura T, Uzawa T, Hokonohara T (2019) Safety and efficacy in actual clinical practice of once-weekly subcutaneous teriparatide for osteoporosis patients with a high fracture risk. Osteoporos Sarcopenia 5:44–50
Acknowledgements
The authors would like to thank the members of the GIOP (Glucocorticoid-induced Osteoporosis) study group for their contributions as co-investigators. This study was sponsored by Asahi Kasei Pharma Corporation.
Author information
Authors and Affiliations
Contributions
All authors contributed to the study conception and design. Data collection was performed by IT and HO. Data analysis was performed by SS and HO. The draft of the manuscript was written by IT, SS, and HO. All authors reviewed and commented on previous versions of the manuscript. The final manuscript was edited by IT and HO, and all authors read and approved the final version of manuscript.
Corresponding author
Ethics declarations
Conflicts of interest
I.Tanaka has received speaking fees, and/or honoraria from Asahi Kasei Pharma, Astellas Pharma, Chugai Pharmaceutical, Daiichi Sankyo, Eisai, Eli Lilly Japan, Mitsubishi Tanabe Pharma, MSD, Ono Pharmaceutical, Pfizer Japan, Takeda Pharmaceutical, Taisho Pharma, and Teijin Pharma. Y. Tanaka has received speaking fees and/or honoraria from AbbVie, Astellas Pharma, Bristol-Myers Squibb, Chugai Pharmaceutical, Daiichi Sankyo, Eisai, Eli Lilly Japan, Janssen Pharmaceutical, Mitsubishi Tanabe Pharma, Novartis Pharma, Pfizer Japan, Takeda Pharmaceutical, Teijin Pharma, and YL Biologics and has received research grants from Asahi Kasei Pharma, Bristol-Myers Squibb, Chugai Pharmaceutical, Daiichi Sankyo, Eisai, Mitsubishi Tanabe Pharma, Ono Pharmaceutical, Sanofi, Takeda Pharmaceutical, and UCB Japan. S. Soen has received consulting fees, speaking fees, and/or honoraria from Asahi Kasei Pharma, Astellas Pharma, Chugai Pharmaceutical, Daiichi Sankyo, Eisai, Eli Lilly Japan, MSD, Ono Pharmaceutical, Pfizer Japan, Takeda Pharmaceutical, and Teijin Pharma. H. Oshima has no conflicts of interest.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
About this article
Cite this article
Tanaka, I., Tanaka, Y., Soen, S. et al. Efficacy of once-weekly teriparatide in patients with glucocorticoid-induced osteoporosis: the TOWER-GO study. J Bone Miner Metab 39, 446–455 (2021). https://doi.org/10.1007/s00774-020-01171-5
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00774-020-01171-5