Abstract
Introduction
Disuse-induced bone loss is caused by a suppression of osteoblastic bone formation and an increase in osteoclastic bone resorption. There are few data available for the effects of environmental conditions, i.e., atmospheric pressure and/or oxygen concentration, on osteoporosis. This study examined the effects of mild hyperbaric oxygen at 1317 hPa with 40% oxygen on unloading-induced osteoporosis.
Materials and methods
Eighteen 8-week old male Wistar rats were randomly divided into three groups: the control for 21 days without unloading and mild hyperbaric oxygen (NOR, n = 6), the unloading for 21 days and recovery for 10 days without mild hyperbaric oxygen (HU + NOR, n = 6), and the unloading for 21 days and recovery for 10 days with mild hyperbaric oxygen (HU + MHO, n = 6).
Results
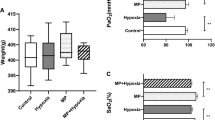
The cortical thickness and trabecular bone surface area were decreased in the HU + NOR group compared to the NOR group. There were no differences between the NOR and HU + MHO groups. Osteoclast surface area and Sclerostin (Sost) mRNA expression levels were decreased in the HU + MHO group compared to the HU + NOR group. These results suggested that the loss of the cortical and trabecular bone is inhibited by mild hyperbaric oxygen, because of an inhibition of osteoclasts and enhancement of bone formation with decreased Sost expression.
Conclusions
We conclude that exposure to mild hyperbaric oxygen partially protects from the osteoporosis induced by hindlimb unloading.




Similar content being viewed by others
References
Raisz LG (2005) Pathogenesis of osteoporosis: concepts, conflicts, and prospects. J Clin Investig 115:3318–3325
Nagaraja MP, Jo H (2014) The role of mechanical stimulation in recovery of bone loss-high versus low magnitude and frequency of force. Life (Basel) 4:117–130
Fujita K, Roforth MM, Demaray S, McGregor U, Kirmani S, McCready LK, Peterson JM, Drake MT, Monroe DG, Khosla S (2014) Effects of estrogen on bone mRNA levels of sclerostin and other genes relevant to bone metabolism in postmenopausal women. J Clin Endocrinol Metab 99:E81–E88
Metzger CE, Brezicha JE, Elizondo JP, Narayanan SA, Hogan HA, Bloomfield SA (2017) Differential responses of mechanosensitive osteocyte proteins in fore- and hindlimbs of hindlimb-unloaded rats. Bone 105:26–34
Leblanc AD, Schneider VS, Evans HJ, Engelbretson DA, Krebs JM (1990) Bone mineral loss and recovery after 17 weeks of bed rest. J Bone Miner Res 5:843–850
Iwamoto J, Takeda T, Sato Y (2005) Effect of treadmill exercise on bone mass in female rats. Exp Anim 54:1–6
Berg HE, Eiken O, Miklavcic L, Mekjavic IB (2007) Hip, thigh and calf muscle atrophy and bone loss after 5-week bed rest inactivity. Eur J Appl Physiol 99:283–289
Klein-Nulend J, Bacabac RG (2012) Bone adaptation and regeneration—new developments. Int J Mod Phys Conf Ser 17:34–43
Bergstrom I, Isaksson H, Koskela A, Tuukkanen J, Ohlsson C, Andersson G, Windahl SH (2018) Prednisolone treatment reduces the osteogenic effects of loading in mice. Bone 112:10–18
Ishihara A, Fujino H, Nagatomo F, Takeda I, Ohira Y (2008) Gene expression levels of heat shock proteins in the soleus and plantaris muscles of rats after hindlimb suspension or spaceflight. J Physiol Sci 58:413–417
Zhang YN, Shi WG, Li H, Hua JR, Feng X, Wei WJ, Wang JF, He JP, Lei SW (2018) Bone loss induced by simulated microgravity, ionizing radiation and/or ultradian rhythms in the hindlimbs of rats. Biomed Environ Sci 31:126–135
Falcai MJ, Zamarioli A, Leoni GB, de Sousa Neto MD, Volpon JB (2015) Swimming activity prevents the unloading induced loss of bone mass, architecture, and strength in rats. Biomed Res Int 2015:507848
Li B, Liu J, Zhao J, Ma JX, Jia HB, Zhang Y, Xing GS, Ma XL (2017) LncRNA-H19 modulates Wnt/beta-catenin signaling by targeting Dkk4 in hindlimb unloaded rat. Orthop Surg 9:319–327
Peres-Ueno MJ, Stringhetta-Garcia CT, Castoldi RC, Ozaki GAT, Chaves-Neto AH, Dornelles RCM, Louzada MJQ (2017) Model of hindlimb unloading in adult female rats: characterizing bone physicochemical, microstructural, and biomechanical properties. PLoS ONE 12:e0189121
Ohira Y, Tanaka T, Yoshinaga T, Kawano F, Nomura T, Nonaka I, Allen DL, Roy RR, Edgerton VR (2001) Ontogenetic, gravity-dependent development of rat soleus muscle. Am J Physiol Cell Physiol 280:C1008–1016
Bloomfield SA, Allen MR, Hogan HA, Delp MD (2002) Site- and compartment-specific changes in bone with hindlimb unloading in mature adult rats. Bone 31:149–157
Shirazi-Fard Y, Kupke JS, Bloomfield SA, Hogan HA (2013) Discordant recovery of bone mass and mechanical properties during prolonged recovery from disuse. Bone 52:433–443
Gill AL, Bell CNA (2004) Hyperbaric oxygen: its uses, mechanisms of action and outcomes. QJM Int J Med 97:385–395
Thom SR (2009) Oxidative stress is fundamental to hyperbaric oxygen therapy. J Appl Physiol (1985) 106:988–995
Oter S, Korkmaz A, Topal T, Ozcan O, Sadir S, Ozler M, Ogur R, Bilgic H (2005) Correlation between hyperbaric oxygen exposure pressures and oxidative parameters in rat lung, brain, and erythrocytes. Clin Biochem 38:706–711
Lima MA, Farage L, Cury MC, Bahamad FJ (2014) Update on middle ear barotrauma after hyperbaric oxygen therapy-insights on pathophysiology. Int Arch Otorhinolaryngol 18:204–209
Ishihara A, Nagatomo F, Fujino H, Kondo H (2014) Exposure to mild hyperbaric oxygen increases blood flow and resting energy expenditure but not oxidative stress. J Sci Res Rep 3:1886–1896
Takemura A, Roy RR, Yoshihara I, Ishihara A (2017) Unloading-induced atrophy and decreased oxidative capacity of the soleus muscle in rats are reversed by pre- and postconditioning with mild hyperbaric oxygen. Physiol Rep 5:e13353
Ishihara A, Kawano F, Okiura T, Morimatsu F, Ohira Y (2005) Hyperbaric exposure with high oxygen concentration enhances oxidative capacity of neuromuscular units. Neurosci Res 52:146–152
Matsumoto A, Nagatomo F, Yasuda K, Tsuda K, Ishihara A (2007) Hyperbaric exposure with high oxygen concentration improves altered fiber types in the plantaris muscle of diabetic Goto-Kakizaki rats. J Physiol Sci 57:133–136
Nakaoka D, Sugimoto T, Kaji H, Kanzawa M, Yano S, Yamauchi M, Sugishita T, Chihara K (2001) Determinants of bone mineral density and spinal fracture risk in postmenopausal Japanese women. Osteoporos Int 12:548–554
Kaji H (2016) Effects of myokines on bone. BoneKEy Rep 5:826
Nagatomo F, Fujino H, Kondo H, Suzuki H, Kouzaki M, Takeda I, Ishihara A (2011) PGC-1alpha and FOXO1 mRNA levels and fiber characteristics of the soleus and plantaris muscles in rats after hindlimb unloading. Histol Histopathol 26:1545–1553
Ellman R, Spatz J, Cloutier A, Palme R, Christiansen BA, Bouxsein ML (2013) Partial reductions in mechanical loading yield proportional changes in bone density, bone architecture, and muscle mass. J Bone Miner Res 28:875–885
Globus RK, Morey-Holton E (2016) Hindlimb unloading: rodent analog for microgravity. J Appl Physiol (1985) 120:1196–1206
Ziambaras K, Civitelli R, Papavasiliou SS (2005) Weightlessness and skeleton homeostasis. Hormones (Athens) 4:18–27
Kondo H, Ezura Y, Nakamoto T, Hayata T, Notomi T, Sorimachi H, Takeda S, Noda M (2011) MURF1 deficiency suppresses unloading-induced effects on osteoblasts and osteoclasts to lead to bone loss. J Cell Biochem 112:3525–3530
Stein GS, Lian JB (1993) Molecular mechanisms mediating proliferation/differentiation interrelationships during progressive development of the osteoblast phenotype. Endocr Rev 14:424–442
Bikle DD, Harris J, Halloran BP, Morey-Holton E (1994) Altered skeletal pattern of gene expression in response to spaceflight and hindlimb elevation. Am J Physiol 267:E822–E827
Hsu H, Lacey DL, Dunstan CR, Solovyev I, Colombero A, Timms E, Tan HL, Elliott G, Kelley MJ, Sarosi I, Wang L, Xia XZ, Elliott R, Chiu L, Black T, Scully S, Capparelli C, Morony S, Shimamoto G, Bass MB, Boyle WJ (1999) Tumor necrosis factor receptor family member RANK mediates osteoclast differentiation and activation induced by osteoprotegerin ligand. Proc Natl Acad Sci USA 96:3540–3545
Kong YY, Yoshida H, Sarosi I, Tan HL, Timms E, Capparelli C, Morony S, Oliveira-dos-Santos AJ, Van G, Itie A, Khoo W, Wakeham A, Dunstan CR, Lacey DL, Mak TW, Boyle WJ, Penninger JM (1999) OPGL is a key regulator of osteoclastogenesis, lymphocyte development and lymph-node organogenesis. Nature 397:315–323
Balemans W, Ebeling M, Patel N, Van Hul E, Olson P, Dioszegi M, Lacza C, Wuyts W, Van Den Ende J, Willems P, Paes-Alves AF, Hill S, Bueno M, Ramos FJ, Tacconi P, Dikkers FG, Stratakis C, Lindpaintner K, Vickery B, Foernzler D, Van Hul W (2001) Increased bone density in sclerosteosis is due to the deficiency of a novel secreted protein (SOST). Hum Mol Genet 10:537–543
Li X, Ominsky MS, Niu QT, Sun N, Daugherty B, D'Agostin D, Kurahara C, Gao Y, Cao J, Gong J, Asuncion F, Barrero M, Warmington K, Dwyer D, Stolina M, Morony S, Sarosi I, Kostenuik PJ, Lacey DL, Simonet WS, Ke HZ, Paszty C (2008) Targeted deletion of the sclerostin gene in mice results in increased bone formation and bone strength. J Bone Miner Res 23:860–869
ten Dijke P, Krause C, de Gorter DJ, Lowik CW, van Bezooijen RL (2008) Osteocyte-derived sclerostin inhibits bone formation: its role in bone morphogenetic protein and Wnt signaling. J Bone Jt Surg Am 90(Suppl 1):31–35
Macias BR, Swift JM, Nilsson MI, Hogan HA, Bouse SD, Bloomfield SA (2012) Simulated resistance training, but not alendronate, increases cortical bone formation and suppresses sclerostin during disuse. J Appl Physiol 112:918–925
Robling AG, Niziolek PJ, Baldridge LA, Condon KW, Allen MR, Alam I, Mantila SM, Gluhak-Heinrich J, Bellido TM, Harris SE, Turner CH (2008) Mechanical stimulation of bone in vivo reduces osteocyte expression of Sost/sclerostin. J Biol Chem 283:5866–5875
Krishnan V, Bryant HU, Macdougald OA (2006) Regulation of bone mass by Wnt signaling. J Clin Investig 116:1202–1209
Semenov M, Tamai K, He X (2005) SOST is a ligand for LRP5/LRP6 and a Wnt signaling inhibitor. J Biol Chem 280:26770–26775
Leach RM, Rees PJ, Wilmshurst P (1998) Hyperbaric oxygen therapy. BMJ 317:1140–1143
Bettis T, Kim BJ, Hamrick MW (2018) Impact of muscle atrophy on bone metabolism and bone strength: implications for muscle–bone crosstalk with aging and disuse. Osteoporos Int 29:1713–1720
Brotto M, Johnson ML (2014) Endocrine crosstalk between muscle and bone. Curr Osteoporos Rep 12:135–141
Mera P, Laue K, Ferron M, Confavreux C, Wei J, Galan-Diez M, Lacampagne A, Mitchell SJ, Mattison JA, Chen Y, Bacchetta J, Szulc P, Kitsis RN, de Cabo R, Friedman RA, Torsitano C, McGraw TE, Puchowicz M, Kurland I, Karsenty G (2016) Osteocalcin signaling in myofibers is necessary and sufficient for optimum adaptation to exercise. Cell Metab 23:1078–1092
Handschin C, Spiegelman BM (2006) Peroxisome proliferator-activated receptor gamma coactivator 1 coactivators, energy homeostasis, and metabolism. Endocr Rev 27:728–735
Puigserver P, Wu Z, Park CW, Graves R, Wright M, Spiegelman BM (1998) A cold-inducible coactivator of nuclear receptors linked to adaptive thermogenesis. Cell 92:829–839
Wu Z, Puigserver P, Andersson U, Zhang C, Adelmant G, Mootha V, Troy A, Cinti S, Lowell B, Scarpulla RC, Spiegelman BM (1999) Mechanisms controlling mitochondrial biogenesis and respiration through the thermogenic coactivator PGC-1. Cell 98:115–124
Colaianni G, Lippo L, Sanesi L, Brunetti G, Celi M, Cirulli N, Passeri G, Reseland J, Schipani E, Faienza MF, Tarantino U, Colucci S, Grano M (2018) Deletion of the transcription factor PGC-1alpha in mice negatively regulates bone mass. Calcif Tissue Int 103:638–652
Kanazawa I, Takeno A, Tanaka KI, Notsu M, Sugimoto T (2018) Osteoblast AMP-activated protein kinase regulates postnatal skeletal development in male mice. Endocrinology 159:597–608
Yokomoto-Umakoshi M, Kanazawa I, Takeno A, Tanaka K, Notsu M, Sugimoto T (2016) Activation of AMP-activated protein kinase decreases receptor activator of NF-κB ligand expression and increases sclerostin expression by inhibiting the mevalonate pathway in osteocytic MLO-Y4 cells. Biochem Biophys Res Commun 469:791–796
Acknowledgements
This work was supported by the Japan society for the promotion of science (Project number 17J02040). The authors are grateful to Dr. Takahiro Yamashita for technical assistance with the experiments.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare no conflicts of interest.
Ethical approval
This study was approved by the Ethics Committee for Human and Animal Research of the Graduate School of Human and Environmental Studies of Kyoto University (Approval number: 30-A-4). All experimental and animal care procedures were conducted in accordance with the Guiding Principles for the Care and Use of Animals in the Field of Physiological Science (The Physiological Society of Japan, 2015).
Informed consent
Informed consent was not required for this type of study.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
About this article
Cite this article
Takemura, A., Pajevic, P.D., Egawa, T. et al. Effects of mild hyperbaric oxygen on osteoporosis induced by hindlimb unloading in rats. J Bone Miner Metab 38, 631–638 (2020). https://doi.org/10.1007/s00774-020-01100-6
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00774-020-01100-6