Abstract
Background
Hemodialysis arteriovenous fistulas (AVFs) are inconstantly used primarily due to problems with maturation, early thrombosis and patient nonacceptance. An endovascular approach to fistula creation without open surgery offers another hemodialysis vascular access option.
Methods
Published studies as well as our own clinical experience and remarkable single center cases are analyzed. Basic literature is reported and expert opinions are discussed.
Results
AVFs with fused anastomoses (Avenu Medical, Ellipsys) were created in 95.0% (102/107) of patients. Primary flow and diameter endpoints were achieved in 86.0% (92/107) of patients. In the NEAT study (Novel Endovascular Access Trial, using the everlinQ 6 Fr system), 80 patients were enrolled. In the EASE study and in all of our patients (access from the wrist) using the novel 4 Fr everlinQ system, an endoAVF was successfully created. Due to the unique anatomy and vessels used to create endoAVFs, those studies were single-arm studies without a surgical comparator.
Conclusions
An endoAVF can be reliably created using different catheter-based systems, without open surgery and with minimal complications. EndoAVFs can be successfully used for hemodialysis and demonstrated a high 12-months cumulative patency in several studies. In the future, it may be an alternative for achieving AVFs for hemodialysis patients in need of a vascular access.
Zusammenfassung
Hintergrund
Chirurgisch angelegte arteriovenöse (AV-)Fisteln zur Hämodialyse entwickeln sich gelegentlich zögerlich oder reifen nach ihrer Anlage nur unzureichend aus. Dazu kommen Frühverschlüsse, überwiegend aufgrund lokaler anastomosennaher Thrombosen. Die Akzeptanz der terminal niereninsuffizienten Patienten für wiederholte chirurgische Prozeduren ist gering. Die endovaskuläre Anlage der AV-Fisteln, ohne offen operativen Eingriff, kann eine Alternative darstellen.
Methode
Wir berichten über unsere eigenen klinischen Erfahrungen, die aktuelle Studienlage und den direkten internationalen Austausch der Anwender der verschiedenen technischen Systeme.
Ergebnisse
Die „Fused-anastomoses-Technik“ (Ellipsys®, Avenu Medical, San Juan Capistrano, CA, USA) war in 95,0 % (102/107) bei der Anlage von endovaskulären AV-Fisteln (endoAVF) erfolgreich. In der NEAT-Studie („Novel Endovascular Access Trial“, everlinQ™ 6Fr-System, TVA medical, Austin, TX, USA) wurden 80 Patienten eingeschlossen. In der EASE-Studie („Endovascular Access System Enhancement“) und bei unseren Patienten mit radialem Zugang konnte mit dem neuen 4Fr-everlinQ™-System bei allen Patienten erfolgreich eine endoAVF angelegt werden. Aufgrund der besonderen anatomischen Anforderungen sind diese Studien durch ihren Single-arm-Charakter und die fehlende chirurgische Vergleichsgruppe in der Aussage noch limitiert.
Schlussfolgerung
EndoAVF können mit hoher Erfolgsquote und niedriger Komplikationsrate angelegt werden. Die erwähnten Studien zeigen auch nach 12 Monaten eine gute funktionelle primäre und kumulative Offenheitsrate. EndoAVF stellen, bei zu erwartender weiterer technischer Entwicklung und Verbesserung der Kathetersysteme, in der Zukunft eine mögliche Alternative zu chirurgischen AV-Fisteln dar.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
The prevalence of end-stage renal disease (ESRD) is increasing globally which imposes a huge economic burden on the healthcare system due to a tremendous logistic and economical effort. In order to replace the kidney function by hemodialysis a vascular access is required. The gold standard which has remained unchanged for over 50 years is still the surgical creation of a native arteriovenous (AV) fistula. New endovascular techniques for creation of AV access can provide a sustainable potential for improvement of shunt placement.
The chance for patients with progressive renal insufficiency or renal failure of receiving a donor organ is constantly decreasing, as the number of available kidney donors is significantly lower than the growing incidence of end-stage renal disease (ESRD) [12, 19]. The use of an AV fistula (shunt) is the most effective way to perform hemodialysis with the least amount of complications as has been shown in many studies and the resulting guidelines [21, 24]; however, patients with a matured AV fistula not only need to undergo dialysis three times per week but often also need to undergo multiple re-interventions to optimize or maintain the vascular access [1].
Current problem situation
In 1966 Brescia et al. came up with a revolutionary approach, which was an enrichment for all players involved in the process, when they described the connection of an artery and a superficial vein to develop a high-quality vascular access for patients with ESRD and the need to receive dialysis. [3]. Since then, vascular medicine has further developed in parallel to neighboring disciplines. The use of constantly improving imaging and technologically mature intervention materials, such as catheters, coils, endoanchors and endoscopes, enables the use of minimally invasive vascular surgery for many diseases that were previously only treatable with open surgery (see, for example, the development in the treatment of abdominal aortic aneurysms [15]). Despite these achievements, not many people would have believed that hemodialysis access could be done without open surgery a decade ago.
Patients have a constantly decreasing chance of receiving a donor organ
The surgical placement of an AV access alone is very demanding and is subject to errors. After surgical exposure of the vessels and dissection of the artery and the vein, an anastomosis without torque and twist is required. Even with a highly precise technique, there may be vessel irritation, spasms, twist and turn phenomena or neointimal hyperplasia, which can all lead to early closure or disturbed maturation [1, 24]. In addition, an intensive preoperative evaluation and selection of the region for anastomosis is essential for the success of a shunt placement [10, 20]. Furthermore, these patients are usually older with multiple comorbidities and the poor vascular conditions result in only a few treatment options. All these factors result in low primary shunt maturation with high failure and/or high re-intervention rates [24].
Unfortunately, there are not many scientific studies in the field of AV access and the variations in the type of access, operative techniques and endpoints also make it difficult to compare the results of these studies [1]. In Germany the majority of surgical shunt procedures are carried out outside university facilities, mostly in specialized centers with predominantly little access to scientific resources. Another structural difficulty in the treatment of dialysis patients with ESRD is the variety of disciplines participating in treatment and in addition to nephrologists (mostly in private practices), vascular surgeons, angiologists and radiologists are also involved that occasionally leads to competing interests among the treating physicians. Combined with an already initiated certification process for AV fistula access centers [8], medical and technical progress and new methods of shunt surgery have the potential to address many of these problems and also to reduce the economic burdens [18].
The innovative endovascular approach
There are essentially two companies pursuing an endovascular AV fistula creation technology. Both systems are based on a similar concept, such as:
-
Vessels are not clamped, not mobilized and not dissected and are not anastomosed by sutures.
-
The AV anastomosis is located in the deep vascular system, where the artery is usually accompanied by one or two concomitant veins.
-
The AV anastomosis is located in the proximal forearm, close or slightly distal to the perforating vein, which is necessary for this procedure.
-
The AV anastomosis is created with heat/radiofrequency through an endovascular catheter system.
An endovascular AV fistula is placed in the deep vein close to the cubital fossa
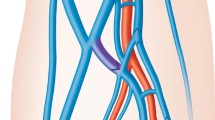
Comparable with traditional surgical approaches, a preprocedural ultrasound assessment to evaluate the vascular status is mandatory in both innovative procedures. The decisive factor for creation of an endovascular AV fistula (endoAVF) is the assessment of the perforator, the vein close to the cubital fossa that connects the deep system with the superficial venous system (Fig. 1). The adequate diameter should be approximately 2 mm and as straight a configuration as possible to either the cephalic or basilic vein is favorable. The perforating vein has multiple anatomical variations and can also be present as a duplication (Fig. 2). In addition, analogue to surgical planning, the superficial veins should have a minimum diameter of 2 mm along the whole length.
An endoAVF is created in the area of the cubital fossa in the deep vascular system, either between the ulnar artery and the accompanying vein or the radial artery and radial vein. These accompanying veins are connected to the perforating vein at the level of the cubital fossa so that a direct outflow to the superficial system is possible [2].
The most comparable surgical procedure in the same anatomical localization is the Gracz fistula, where the brachial artery is connected with the venous perforator in order to achieve maturation of both the cephalic and the basilic veins [5]. Surgical variations, such as connecting the radial or ulnar artery with the perforating vein have also been described in the literature [9]. Fig. 1 shows a simplified typical anatomical illustration of the vascular system of the right arm. Deviations, such as differences in vessel diameter, high brachial bifurcations (approximately 10%) or nonexistent perforating veins are identified prior to the endoAVF procedure through the ultrasound/duplex examination.
EverlinQ™/WavelinQ system
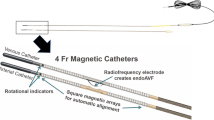
The first generation everlinQ™ system (TVA Medical, Austin, TX, USA) had two 6 Fr catheters, which were inserted via the upper arm into the vascular system (artery and accompanying vein). Since the end of 2017 a CE-approved 4 Fr device has replaced the old 6 Fr system in Europe. Both catheters are equipped with very potent magnets in order to adapt the target vessels (artery and vein) for creation of the anastomosis. The arterial device has a small ceramic saddle between the magnets (Fig. 3) and the venous device has an arcuate electrode that ideally should perfectly sit in the ceramic saddle.
Preoperative ultrasound is used to determine whether an ulnar or radial anastomosis is to be placed. Decisive are here the caliber of the vessels and the configuration of the perforating vein. At the same time the access site is determined. The newer 4 Fr system enables a wrist access either from the radial or the ulnar side if the diameters of the vessel are sufficient, otherwise access via the brachial vessels is performed. As in the case of the Ellipsys system (Avenu Medical, San Juan Capistrano, CA, USA) a venous puncture of the superficial upper arm veins with subsequent navigation via the perforating vein into the deep venous system is possible.
The procedure usually starts with a light sedation of the patient followed by local anesthesia at the puncture site. The arm to be treated is placed and fixed on a carbon arm board transparent to imaging to prevent movement due to myoclonus triggered by radiofrequency impulse-induced neurostimulation during energy delivery (more frequent and more severe in creation of an ulnar fistula due to proximity of the medial nerve). Following duplex sonography-guided puncture 0.014″ guidewires are inserted in both vessels up to the planned site of the anastomosis. The two catheters are positioned via these wires and fluoroscopic imaging is used to check the correct positioning and coaptation.
The preset high-frequency (HF) energy of 60 W is applied by manual control and a preprogrammed timer for 0.7 s to create the endoAVF. The created anastomosis always has the same dimensions with the size and length of the saddle on the arterial catheter (approximately 4 mm in length and 1 mm wide). No implant or suture material remains at the endoAVF site.
The introduction of the 4 Fr system more than halved the radiation exposure
The result is documented by angiography. In these procedures a pronounced intraoperative vessel spasm is often seen after HF application (see Fig. 4), possibly triggered by thermal effects, small hematomas or electrical stimulation that can usually no longer be detected in the early postoperative phase. In most procedures a coil embolization of the deep brachial vein (Fig. 2) is performed proximal to the anastomosis and the perforating vein to increase the outflow into the superficial venous system. The anticipated venous congestion triggered by this procedure has so far only been seen in a mild form in 1 of the 26 patients treated so far. Other publications have also not reported comparable problems regarding this issue [6, 7, 11, 14, 17].
Although many steps in this procedure can be carried out by ultrasound guidance, angiographic imaging is still required. In the series with the 6 Fr system, the average area-dose product was 2.15 Gy/cm2 (minimum 0.82 Gy/cm2, maximum 4.89 Gy/cm2, median 1.77 Gy/cm2). The introduction of the 4 Fr system more than halved the radiation exposure with an average area-dose product of 0.91 Gy/cm2 (minimum 0.41 Gy/cm2, maximum 1.74 Gy/cm2, median 0.78 Gy/cm2). Radosa et al. reported values almost 20 times higher with the same procedure (6 Fr) where a mean area-dose product of 46.4 Gy/cm2 was reported [16], which is a higher exposure dose than in diagnostic coronary angiography (27.2 Gy/cm2) and percutaneous transluminal angioplasty (PTA, 25 Gy/cm2) but a lower exposure dose than in percutaneous coronary interventions (56.8 Gy/cm2) or a PTA with stent implantations (97 Gy/cm2) [13].
The differences are so obvious that they cannot simply be explained by the intensive intraoperative use of ultrasound alone.
Clinical results of the everlinQ™/WavelinQ system
The first clinical results of this procedure have been compiled in the FLEX study, a prospective clinical evaluation of total vascular access (TVA). Technical success was achieved in 32 out of 33 cases (97.0%). In 96.0% of the patients, the shunt was matured after 3 months and could be used for dialysis [17]. In the multinational and multicenter novel endovascular access trial (NEAT) follow-up study, 80 patients (20 roll in, 60 in the analysis) were treated with the 6Fr system and analyzed. A high technical success rate (98%) and usability of the fistula (functional patency 87%) was achieved [11]. The EASE study reporting data from the newer 4Fr system has only been presented at the time of publication of this article (a publication is in preparation). All patients successfully received an endoAVF ([22]; Table 1).
Ellipsys® system
The Ellipsys® system from Avenu Medical (Fig. 5) creates an endoAVF using a single 6 Fr catheter that thermally connects the vessels. The suitability and the strategic procedure for this system are also determined sonographically. Dr. A. Mallios, with great personal experience in Paris (personal communication) and Avenu Medical, describe the procedures that were performed guided only by ultrasound and therefore without radiation exposure and contrast media. This procedure starts with a puncture of a superficial vein (either cephalic or basilic vein) on the upper arm to navigate from there through the perforator to the deep venous system. With the aid of ultrasound the radial artery is localized from the vein and punctured and a guidewire is placed distally in the artery. The Ellipsys® device is inserted via the 0.014″ guidewire and advanced to the defined target region. The catheter has two contact plates, of which one is placed in the vein and the other in the artery. The anastomosis is created by electrical impulses of up to 15 s before the system is removed and the success can now be checked with either ultrasound or angiography.
Placement of the endoAVF with the Ellipsys® device. a Puncture of the radial artery via the perforating vein, b placement of the wire and the sheath, c the contact plates grip both vessel walls, d direct current creates the anastomosis. (reproduced with kind permission of Avenu Medical. This figure is not part of the Open Access agreement)
An immediate PTA of the anastomosis and the perforating vein is increasingly being carried out. In this respect there is a significant difference between the two systems: while PTA is possible or even recommended using the Ellipsys® system [14], for the everlinQ™ system a postprocedural PTA cannot be recommended at any time because directly after creation the anastomosis is still too fragile.
Clinical results of the Ellipsys® system
The first published study with the Ellipsys® device showed a technical success rate of 88.0% (23 out of 26 patients). Of these endoAVFs 22 were patent after 6 weeks with an average time to starting dialysis of 108 days. To improve maturation of the shunts, 48% of the patients had to be treated with an additional PTA [6].
These methods potentially provide economic benefits
The second published paper described the American approval study at five centers in the USA. In 95.0% of the patients (102 out of 107 in total) an endoAVF was successfully created. Severe adverse events were not observed and a 2-needle dialysis could be achieved in 88.0% of the patients. In this study additional interventions were also necessary in 99 patients. A total of 205 were necessary to enable maturation of the shunt, including 113 PTAs of the anastomosis in 77 patients, 42 coil embolizations of the deep venous system in 34 patients, 34 cubital vein occlusions were carried out in 33 patients (17 ligatures and 17 embolizations), side branches of accessory superficial veins were embolized 40 times in 37 patients and 28 surgical transpositions. In order to maintain primary and functional patency another 66 interventions, including 51 PTAs, 10 embolizations and 8 stents (multiple mentions) were performed in 36 patients. Among these a functional patency of 98.4%, 98.4% and 92.3% was described after 90, 180 and 360 days, respectively [7]. In 2018 Mallios et al. reported similar results in 34 patients, with a technical success of 96% and a cumulative patency rate of 92% over an average follow-up period of 141 days (53–229 days) [14].
Both technologies are currently being further evaluated in postcommercial registries and/or monocentric surveys in Europe. The aim is not only to ensure continuous proof of the feasibility and effectiveness of the methods but also to examine the economic benefits that these methods potentially provide.
Both the Ellipsys® and the everlinQ™ (6 Fr) system devices received U.S. Food and Drug Administration (FDA) approval on 22 June 2018 and are therefore commercially available in the USA [23].
The company TVA Medical was acquired by Becton Dickenson (BD) in July 2018 and within the framework of the integration the catheter system is available under the new name of the WavelinQ endoAVF system.
Economic considerations
Innovative procedures are often criticised for the high costs. Also, with the endoAVF procedures, the initial costs for the catheters are significantly higher compared to surgical AVF creation. In Germany, the everlinQ procedure has a new investigation and treatment method (NUB) status 1 since 2016, which allows hospitals to negotiate a dedicated reimbursement with the health insurance companies for this procedure. This status 1 is only granted when the cost deficit for such new procedures with the currently available codes within in the diagnosis-related groups (DRG) system is so large that an additional budget must be negotiated. Since 2018 both procedures can be documented with an individual procedural code (codes 8‑83c.c and 8‑83c.d) but only the everlinQ method still has the NUB status 1 [4].
Introducing a new medical technical device onto the market requires immense financial and administrative efforts in order to make the new product marketable and takes many years. The selling price must also justify the treatment in the healthcare system from a cost efficiency perspective. The everlinQ device was analyzed under this aspect in 2017. Yang et al. [25] compared the results of the NEAT study with an historical cohort from American insurance data that showed pretty much the same patient demographics. More than 1 year after surgical and endovascular creation of an AV fistula the complications, re-interventions and central vein catheter (CVC) exposure times were compared. Significantly lower rates of angioplasty, thrombectomy, surgical revision, CVC, grafts, new fistula creations and access-related infections in the endoAVF group led to a saving benefit of over US$11,000 compared to conventional surgical creation of AV access.
Conclusion
An EndoAVF can even now be created with a higher success rates and low complication rates. Under study conditions and with appropriate patient selection, both commercially available catheter systems demonstrated high functional primary and cumulative patency rates even after 12 months. It appears that the marginally lower AV fistula flow volumes distributed over a greater outflow area bring positive effects to the long-term function and secondary degeneration of the access stretch. In patients who no longer have superficial veins available due to multiple intravenous access points, drug abuse, skin burns or a long history of dialysis, an initial endovascular anastomosis of deep veins and arteries before a planned elevation and/or transposition is a very attractive scenario to add a native option to the AV access repertoire.
An EndoAVF can now be created with a higher success rates and low complication rates
The progressive technical development of catheter systems extends the possibilities for endovascular shunt placement and new less invasive options will continue to be introduced. Germany has a great standing for vascular treatment, so it is the perfect country to attract new technologies such as endoAVF which are minimally invasive, more cost-effective and lead to better quality of life for patients and ultimately to avoid central venous transient or long-term catheterization in hemodialysis patients. Nephrologists and also well-informed patients are looking for alternatives to the classical surgical AV fistula placement and the gruelling revision interventions.
Practical conclusion
-
For the standard placement of the endoAVF the perforating vein is indispensable.
-
Two procedures are currently commercially available.
-
Ultrasound and a high-quality radiological imaging are necessary.
-
The technical success rates in the studies were >90%.
-
Endovascular experience is recommended for the procedure.
-
The high material costs require an additional remuneration (NUB).
-
The patient acceptance is good.
-
The different shunt characteristics necessitate a close cooperation with the referring nephrologists.
Literatur
Al-Jaishi A, Oliver M, Thomas S, Lok C et al (2014) Patency rates of the arteriovenous fistula for hemodialysis: a systematic review and meta-analysis. Am J Kidney Dis 6(3):464–478
Berge MT, Yo T, Kerver A, Smet A, Kleinrensink G (2011) Perforating veins: an anatomical approach to arteriovenous fistula performance in the forearm. Eur J Vasc Endovasc Surg 42:103–106
Brescia M, Cimino J, Appel K, Hurwich B (1966) Chronic hemodialysis using venipuncture and a surgically created arteriovenous fistula. N Engl J Med 275:1089–1092
Deutsches Institut für medizinische Dokumentation und Information. (2018) www.dimdi.de
Gracz K, Ing T, Soung L, Armbruster K, Seim S, Merkel F (1977) Proximal forearm fistula for maintenance hemodialysis. Kidney Int 11:71–75
Hull J, Elizondo-Riojas G, Bishop W, Voneida-Reyna Y (2017) Thermal resistance anastomosis device for the percutaneous creation of arteriovenous fistulae for hemodialysis. J Vasc Interv Radiol 28:380–387
Hull J, Jennings W, Cooper R, Waheed U, Schaefer M, Narayan R (2017) The pivotal multicenter trial of ultrasound-guided percutaneous arteriovenous fistula creation for hemodialysis access. J Vasc Interv Radiol 29:149–158.e5
Interdisziplinäre Arbeitsgruppe zum Dialysezugang (2017) www.clarcert.com
Konner K (2003) The initial creation of native arteriovenous fistulas: surgical aspects and their impact on the practice of nephrology. Semin Dial 16:291–298
Krönung G (2011) Dialyseshunts. Thieme, Stuttgart
Lok C et al (2017) Endovascular proximal forearm arteriovenous fistula for hemodialysis access: results of the prospective, Multicenter Novel Endovascular Access trial (NEAT). Am J Kidney Dis 70:486–497
Lozano RN (2012) Global and regional mortality from 235 causes of death for 20 age groups in 1990 and 2010: a systematic analysis for the Global Burden of Disease Study 2010. Lancet 380:2095–2128
Majewska N, Blaszak M, Juszkat R, Frankiewicz M, Makalowski M, Majewski W (2011) Patients’ radiation doses during the implantation of stents in carotid, renal, iliac, femoral and popliteal arteries. Eur J Vasc Endovasc Surg 41:372–377
Mallios A, Jennings W, Boura B, Costanzo A, Borquelot P, Combes M (2018) Early results of percutaneous arteriovenous fistula creation with the ellipsys vascular access system. J Vasc Surg. https://doi.org/10.1016/j.jvs.2018.01.036
Parodi JP (1991) Transfemoral intraluminal graft implantation for abdominal aortic aneurysms. Ann Vasc Surg 6:491–499
Radosa C, Radosa JC, Weiss N, Schmidt CS, Werth S, Hofmockel T, Plodeck V, Gatzweiler C, Laniado M, Hoffmann R (2017) Endovascular creation of an arteriovenous fistula (endoAVF) for hemodialysis access: first results. Cardiovasc Intervent Radiol 40:1545–1551
Rajan D, Ebner A, Desai S, Cohn W (2015) Percutaneous creation of an arteriovenous fistula for hemodialysis access. J Vasc Interv Radiol 26(4):484–490. https://doi.org/10.1016/j.jvir.2014.12.018
Schon D (2007) Increasing the use of arteriovenous fistula in hemodialysis: economic benefits and economic barriers. Clin J Am Soc Nephrol 2:268–276
Segerer K, Wanner C (2014) Niere und ableitende Harnwege. Springer, Heidelberg, Berlin
Shenoy S (2009) Surgical anatomy of upper arm: what is needed for AVF planning. J Vasc Access 10:223–232
Sidawy A, Gray R, Henry M, Ascher E et al (2002) Recommended standards for reports dealing with arteriovenous hemodialysis accesses. J Vasc Surg 35:603–610
Steinke, T. (2018). Clinical experience and latest clinical data with the newest EndoAVF technology. Presented at LINC 2018, Feb 1.
U.S. Food & Drug Administration (2018) www.fda.gov
Vascular Access Group (2006) Clinical practice guidelinesfor vascular access. Am J Kidney Dis 48:248–273
Yang S, Lok Ch, Arnold R, Rajan D, Glickman M (2017) Comparison of post-creation procedures and costs between surgical and an endovascular approach to arteriovenous fistula creation. J Vasc Access 18:8–14
Acknowledgements
The authors are grateful to the companies for providing the graphic and image material.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
T. Steinke declares that he received travelling expenses and advisory fees from BD/Bard, TVA Medical, Meritmedical. J. Rieck is an employee of AngioConsult GmbH, which until recently undertook advisory activities for TVA Medical. L. Nuth declares that she has no competing interests.
This article does not contain any studies with human participants or animals performed by any of the authors.
The supplement containing this article is not sponsored by industry.
Additional information
The German version of this article can be found under https://doi.org/10.1007/s00772-018-0466-9.
Rights and permissions
Open Access. This article is distributed under the terms of the Creative Commons Attribution 4.0 International License (http://creativecommons.org/licenses/by/4.0/), which permits unrestricted use, distribution, and reproduction in any medium, provided you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made.
About this article
Cite this article
Steinke, T., Rieck, J. & Nuth, L. Endovascular arteriovenous fistula for hemodialysis access. Gefässchirurgie 24 (Suppl 1), 25–31 (2019). https://doi.org/10.1007/s00772-018-0500-y
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00772-018-0500-y