Abstract
The increasing endovascular treatment of arterial stenoses requires reliable sonographic criteria for the detection of recurrent stenosis. Placement of a stent reduces the vessel lumen and is assumed to lead to greater rigidity, thereby causing more pulsatile blood flow and a higher peak systolic velocity (PSV). In vitro experiments and clinical trials with duplex ultrasound follow-up after percutaneous transluminal angioplasty (PTA) of the internal carotid artery with carotid artery stenting (CAS) provide inconsistent data on the magnitude of the increase in PSV of in-stent restenosis. They range from unchanged PSV up to 30% higher increases in PSV measurement values compared to those established for stenosis grading in native carotid stenoses. In stented leg arteries, studies even show somewhat lower PSV values compared with stenosis in unstented vessels. The option of applying the continuity law, so far not mentioned in the international literature, allows more accurate grading of in-stent restenosis. This method relates the PSV measured at the site of in-stent restenosis to the prestenotic PSV measured within the stent, thereby eliminating the need to take altered hemodynamics in stented arteries versus unstented arteries into account.
Zusammenfassung
Die zunehmende endovaskuläre Therapie arterieller Stenosen erfordert zuverlässige sonographische Kriterien zur Detektion von Rezidivstenosen. Die angenommene erhöhte Rigidität sowie die Lumenreduktion führen nach Stentimplation im Gefäß zu einer erhöhten Pulsatilität und einer erhöhten systolischen Spitzengeschwindigkeit (PSV). Sowohl In-vitro-Versuche als auch Studien mit duplexsonographischer Verlaufskontrolle nach perkutaner transluminaler Angioplastie (PTA) der A. carotis interna mit Stentimplantation (CAS) zeigen jedoch sehr unterschiedlich Angaben über die PSV-Erhöhung bei Rezidivstenosen. Sie reichen von unveränderter PSV bis zu einer Erhöhung um 30 % gegenüber den PSV-Messwerten, die in der Stenosegraduierung nativer Karotisstenosen etabliert sind. Bei gestenteten Beinarterien zeigen Studien sogar etwas geringere PSV-Werte gegenüber Stenosen nativer Arterien. Die bisher in der internationalen Literatur nicht erwähnte Möglichkeit der Stenosegraduierung über das Kontinuitätsgesetz, mit dem Vergleich der prästenotischen, aber im Stent gemessenen PSV mit der PSV in der Rezidivstenose, führt zu einer exakteren Stenosegraduierung, ohne dass dabei die Veränderung der Hämodynamik zwischen nativem und gestentetem Gefäß berücksichtigt werden muss.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Ultrasound follow-up after PTA and internal carotid artery stenting
Background
Percutaneous transluminal angioplasty (PTA) of the internal carotid artery (ICA) with carotid artery stenting (CAS) has evolved to become an effective alternative to carotid thromboendarterectomy (TEA) for the revascularization of high-grade carotid stenosis, particularly in patients at high surgical risk (comorbidities, previous surgery in the neck area, radiotherapy). As in carotid TEA, follow-up is also necessary after CAS, and duplex ultrasound is the method of choice. In-stent recurrent stenosis rates (>70%) are reported to be on average 6% within the first year, and 3–12% within the first 2 years. Reports in the literature, however, put in-stent recurrent stenosis at between 1% and 50% [1,2,3,4,5]. This variation is due to a number of different factors:
-
Definition of recurrent stenosis (degree of stenosis)
-
Patient collective composition
-
Duration of follow-up and stent design
-
Prevalence of predictors for recurrent stenosis in the patient collective
-
Measurement methods (peak systolic velocity, PSV, in duplex ultrasound) to grade stenosis. For example, higher recurrent stenosis rates (up to 32%) for 50% carotid in-stent stenosis have been reported when working with the duplex ultrasound criteria for native, unstented carotid stenosis (>130 cm/s) [2, 4,5,6].
The main duplex ultrasound criterion in the diagnosis of recurrent stenosis is increased systolic PSV; however, it is essential here to clarify whether elevated PSV really is caused by neointima-related recurrent stenosis or by stent-related hemodynamic changes.
The larger multicenter studies comparing recurrent stenosis rates between carotid TEA and CAS also worked with different PSV as threshold velocities as a criterion for relevant recurrent stenosis (>70%): CAVATAS [7], SPACE [8] with >2.1 m/s and CREST [9] with >3 m/s. Differing recurrent stenosis rates were also attributed to this. Therefore, the EVA 3S study [3] took a PSV of >2.1 m/s for >70% recurrent stenosis following carotid TEA and a PSV of >3 m/s following CAS.
Predictors of recurrence
Knowledge of the predictors of recurrent stenosis is an important criterion for determining postoperative follow-up intervals. Several studies revealed primarily diabetes, with its high potential for neointimal proliferation, as well as hyperlipidemia, female sex, nicotine abuse, and a plaque length of over 2 cm [4, 5, 10,11,12,13] as predictors for in-stent recurrent stenosis, but not plaque morphology as determined preoperatively by ultrasound (gray scale median analysis), plaque echogenicity [14, 15], or plaque calcification [16]. The causes of hemodynamically relevant recurrent stenosis primarily include the onset of in-stent neointimal proliferation usually within the first year, as well as atherosclerosis that progresses in the further course with in-stent stenosis due to plaque. Rare, but very early onset stent narrowing can occur due to restoring forces of plaque pressed against the vessel wall.
These cause the stent to be pushed back into the lumen without resulting in deposits occurring primarily in the stent. They generally cause non-treatment relevant lumen reduction that cannot be adequately visualized in radiological follow-up, e.g. digital subtraction angiography (DSA), computed tomography angiography (CTA) and magnetic resonance imaging (MRI), particularly if the stent segment protruding into the vessel lumen is surrounded by contrast medium (Fig. 1; video clip 2).
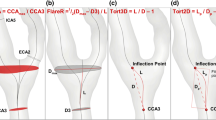
The restoring forces of the plaque at the internal carotid artery (ICA) bifurcation on the wall stent result in a tapered lumen reduction at the end of the stent despite a primarily good stent position. A lumen reduction of approximately 50% corresponds to a duplex ultrasound doubling of an in-stent peak systolic velocity (PSV) from 100 to 210 cm/s at the end of the stent in the narrowed area (according to the continuity law). The arrows indicate the area over which the region of elevated PSV can be determined by shifting the transducer at the same Doppler angle. ECA external carotid artery, CCA common carotid artery
Color duplex ultrasound follow-up intervals following carotid artery stenting
Intravascular stents can be readily evaluated by means of color duplex sonography; the carotid pathway exhibits the same ultrasound conditions as before stent placement and the stent itself can be well-differentiated due to its mesh-like structure. Ultrasound follow-up should be performed at 3 months and then every 6 months in the first 2 years, particularly in patients with the abovementioned predictors for recurrent stenosis. Thereafter, annual follow-up is sufficient in hitherto non-stenosed stent areas. An examination 1 week following stent placement, including determination of post-interventional PSV, makes it easier to grade recurrent stenosis, whereby an increase compared to this baseline (more than double) signals relevant recurrent stenosis [17].
Influence of the stent on hemodynamics
Several publications postulated altered arterial hemodynamics in the stent area following stent placement [18, 19]. Increased vascular stiffness in the stented area should cause increased pulsatility (Fig. 2), with a drop in the diastolic portion in the spectrum and increased PSV. This increase in PSV may be further accentuated by stent-related lumen reduction.
Change in peak systolic velocity (PSV) and pulsatility following stent placement: a stent with morphologically normal flow in the internal carotid artery (ICA) with a PSV of 153 cm/s, which can be inferred for the entire length of the stent. According to studies, this PSV would in actual fact correspond to approximately 30–40% stenosis (the North American Symptomatic Carotid Endarterectomy Trial, NASCET, criterion). Stent rigidity is the cause of the increased pulsatility and elevated PSV. ECA external carotid artery, CCA common carotid artery
In vitro stent placement in an ovine carotid artery without simulated stenosis produced a PSV increase of 22% and, in artificially induced stenosis, a stenosis-dependent increase of 20–30% in stented compared to unstented arteries [18]. In cases where the cause for this is unknown, pseudo-acceleration of PSV due to interference between the ultrasound signal and stent material is assumed; however, the question is then whether this in vitro assumption in the case of stent incorporation with neointimal proliferation is still relevant in vivo. Furthermore, this investigation described a three-fold drop in compliance in the stented area in its in vitro test series. A mismatch between stented and native segments of the artery is also hypothetically postulated [20]. Another study described significantly reduced compliance (ratio of systolic/diastolic diameter changes and systolic/diastolic blood pressure changes) in stented arteries [21]; however, other in vitro experiments failed to detect any relevant, or at most only a slight, increase in flow velocity in stented areas in flow models between unstented and stented vessel areas [22, 23]. Thus, in this experiment, the mean PSV increased by only 6.4% in a stented compared to a non-stented model [22], and in a comparison of different stents, a stent-dependent difference in PSV, partly an increase and partly also a drop in PSV, was observed [23]; however, closed-cell stents in particular exhibit a higher PSV compared to open-cell stents [24]. Thus, in vitro experiments show divergent results and cast doubt on stenosis grading on the basis of absolute PSV values.
Discrepancies in the grading of recurrent stenosis and evidence after CAS
In several studies, follow-up of CAS showed threshold velocities of 150–240 cm/s in >50% stenosis and 300–450 cm/s as the threshold velocity in over 70% (up to 80%) stenosis (Table 1). These predominantly Anglo-American studies carried out exclusively according to the degree of distal stenosis (the North American Symptomatic Carotid Endarterectomy Trial, NASCET, criterion) from 2002 to 2008, led to additional misunderstandings in German-speaking countries in the comparison of threshold velocities in native carotid stenosis, since the threshold velocities of these study results for recurrent stenosis were compared with the degree of local stenosis (European Carotid Surgery Trial, ECST, criterion) used at that time in the German-speaking regions for de novo stenosis [25]. When converted, this grade of local stenosis shows approximately 30% higher PSV values for 50–70% stenosis compared to the degree of distal stenosis (NASCET criterion). According to the NASCET criterion, the main criterion for stenosis is a PSV of 325 cm/s in 70% de novo carotid stenosis [26]. The PSV according to the ECST criterion (local degree of stenosis) was converted at a consensus conference to the NASCET criterion and adjusted from 200 cm/s (ECST criterion) to 300 cm/s [27]. On the other hand, parallel to this discussion process and following further Anglo-American studies, the PSV for native ICA stenosis, according to the NASCET criterion (distal degree of stenosis), was adjusted downwards on the basis of receiver operating characteristic (ROC) curves: PSV > 140 cm/s for >50% stenosis and PSV > 230 cm/s for >70% stenosis.
The same working group, using an ROC curve analysis in an extensive study with a good study design [19], defined the ideal threshold velocity for >30% in-stent recurrent stenosis (NASCET criterion) as a PSV of >150 cm/s, a PSV of 225 cm/s for >50% stenosis, and a PSV of 325 cm/s for >80% stenosis. What is striking, however, is the wide spectrum of PSV values, ranging from 142 cm/s to 256 cm/s (mean PSV of 180 cm/s) for 30–50% stenosis, 201–408 cm/s (mean PSV of 278 cm/s) for 50–80% stenosis, and 58–613 cm/s (mean PSV 403 cm/s) for 80–99% stenosis. The most comprehensive study [36] found a PSV of 175 cm/s as the cut-off for >50% in-stent recurrent stenosis and a PSV of 300 cm/s for >70% stenosis. The increased PSVs in in-stent recurrent stenosis were also confirmed by intravascular ultrasound in a study with a small number of cases [38].
The studies (Table 1) showed similar PSV threshold velocities for in-stent recurrent stenosis >50% and >70% to the original NASCET criterion [26] and as defined in the Interdisciplinary Guidelines Conference [27] for native ICA stenosis (according to the distal degree of stenosis/NASCET criterion); however, compared to the PSV for native ICA stenosis corrected downwards, the threshold velocity for in-stent recurrent stenosis increased by 20–30% [28, 29]. The consequences of the different approaches to stenosis grading is impressively illustrated in two studies, which together put the ideal threshold velocity for de novo ICA stenosis of >50% at 180 or 200 cm/s (NASCET criterion) and for recurrent stenosis after stenting for >50% stenosis at 220 or 240 cm/s [30, 32], i. e., increased by only approximately 10% (−20%).
The weaknesses of number games of this kind with respect to threshold velocities is highlighted in a publication on a small number (6) of cases of recurrent stenosis (in 141 CAS) [37]. On the basis of 5 cases of recurrent stenosis >80% and recurrent stenosis between 50% and 80%, an ideal cut-off of 195 cm/s for >50% stenosis and only 205 cm/s for >80% stenosis was determined. Compared to other literature reports, they recommended by inference that each laboratory develops its own criteria.
Some studies also examined the end-diastolic velocity (EDV) and the ratios of in-stent internal carotid artery to common carotid artery (ratio ICA:CCA) as a possible stenosis criterion for recurrent stenosis (Table 1); however, PSV shows the highest accuracy and the highest correlation compared to CTA or angiography (e. g., DSA) performed as a reference method. In addition, however, these criteria (Table 1) should also be taken into account for equivocal or unclear findings. Furthermore, the secondary criteria known from the diagnosis of native ICA stenosis should also be taken into consideration [25, 27].
The validity of previous retrospective studies is diminished by their small case numbers, although some studies showed follow-up in over 100 patients after CAS. These are usually compared to CTA as a reference method and, depending on the study, only 10–30 patients were compared using the gold standard of angiography for stenosis grading, i. e., only patients with higher-grade recurrent stenosis, often as part of re-intervention (Table 1). Furthermore, stent design, which was not taken into account in many studies, seems to have an impact on the rigidity and thus the rise in PSV following stent placement. For example, closed-cell stents show a significantly higher PSV compared with open-cell stents (mean PSV 115 cm/s versus 93 cm/s, p = 0.003) [22]. This was confirmed in another study [39] (mean PSV 122 cm/s versus 93 cm/s, p = 0.007).
One way to minimize the varying impact of different stent types on PSV is by means of a baseline PSV, which is determined 1 week after stent implantation and forms the basis for further follow-up. For example, a doubling of this PSV over time was interpreted as a sign of relevant stenosis and evaluated with high accuracy [17].
Stenosis grading according to the continuity law
Since the majority of restenotic lesions develop within the stent, some in the mid-third but most at the distal end [40], the stenosis criterion used in peripheral artery diagnosis of a sudden increase in the intrastenotic PSV compared to prestenotic velocity (Figs. 1, 3, 4 and 5) can be used (video clips 1–3). According to the continuity law a ratio (intrastenotic PSV/prestenotic PSV) of >2 in concentric stenosis is an indication of >50% stenosis and a ratio of >4 an indication of >75% stenosis. Using ratios determined in this way, the prestenotic PSV in the stent is measured slightly proximal to the stenotic area (Figs. 4 and 5) and not, as in some studies (Table 1), as the ratio of the in-stent velocity in the ICA to the velocity in the CCA (similar to the diagnosis of native ICA bifurcation stenosis); however, this determination of such a PSV ratio (ICA/CCA) is subject to similar errors as the same stenosis grading used as a secondary criterion for native ICA stenosis: the hemodynamics of the external carotid artery (ECA), which is also supplied by the CCA, are not taken into account and, depending on flow and collateral efficacy of the external circulation as well as on CCA diameter, PSV values vary.
Recurrent stenosis of the internal carotid artery (ICA) due to neointima formation at 1 year following stent placement: color duplex ultrasound shows, morphologically, approximately 50%, long segment narrowing due to neointima in the stent area (a); however, the peak systolic velocity (PSV) is only 143 cm/s in the stenosed area. The PSV measured in-stent but at the ICA bifurcation is 58 cm/s, from which the author obtains a ratio of >2 (b) according to the continuity law, i. e., 50–60% recurrent stenosis; however, the intrastenotic PSV of 143 cm/s is lower than that in Fig. 2 with a non-stenosed, normal ICA (PSV: 153 cm/s) after stenting as an indication of the high variability of PSV measured in-stent. ECA external carotid artery, CCA common carotid artery
a High-grade in-stent recurrent stenosis at the distal end of the stent with a ratio (in-stent intrastenotic/in-stent prestenotic and in the internal carotid artery (ICA) shortly after external carotid artery bifurcation) of >4: intrastenotic peak systolic velocity (PSV) 414 cm/s and prestenotic PSV 97 cm/s. The sudden increase in PSV in the Doppler frequency spectrum was visualized by moving the transducer over the skin in a cranial direction at the same Doppler angle (curved-array transducer; tilted to achieve a Doppler angle of 54°) during continuous recording. b Control angiography with a higher degree of recurrent stenosis at the end of the stent
The author’s own approach to in-stent stenosis grading according to the continuity law: since most in-stent recurrent stenosis occurs along the course of the stent or often at the distal end of the stent, the ratio of peak systolic velocity (PSV; intrastenotic PSV/prestenotic PSV) but in the stent area of the internal carotid artery (ICA) can be graded most accurately according to the continuity law. There is no discussion of stenosis grading according to local or distal stenosis, since the stent levels out fluctuations in the bulge, and the in-stent diameter in the bulge region is comparable to the diameter of the distal ICA. ECA external carotid artery, CCA common carotid artery
The prestenotic (but measured in the stent) PSV of the ICA is a reliable base value for the ratios (Figs. 4 and 5). Since one can assume the same rigidity along the length of the stent, this is of no relevance for this PSV ratio. In addition, systemic factors effecting PSV are circumnavigated, as is a compensatory flow increase in the case of contralateral ICA stenosis. In an initial summary on an as yet small collective of patients, stenosis grading performed according to this method (PSV ratio) in all 9 patients with high-grade stenosis in whom 75% stenosis was determined according to the continuity law with the ratios (>4) described above, was confirmed on subsequent angiography. The PSV in this group was 230–455 cm/s. A total of 18 patients with a ratio of 2–4 had a PSV of 125–280 cm/s [25] in 50–75% stenosis. Here, however, the ratio and not the absolute PSV forms the basis for stenosis grading. There are no further data in the literature on this specially developed measuring method.
The relevance of and need for studies on grading according to the continuity law (prestenotic cross-sectional vessel area/intrastenotic area = intrastenotic PSV/prestenotic PSV) can be called into question, since physical laws that were confirmed in in vitro experiments form the basis of stenosis grading. This ratio (intrastenotic PSV/prestenotic PSV) corresponds closely, from low-grade up to 90% stenosis, in terms of stenosis grade with the values determined by the continuity law, where the degree of stenosis = (1−1/PSV ratio) × 100 [25].
It is important in terms of the measuring method, particularly in bent stents, that the Doppler angle along the vessel is adequately corrected. The investigator-dependence of the method is evident in the collection of measurement data.
Plaque configuration
It is important to note, however, that in any stenosis grading method concentric plaques are of greater hemodynamic relevance than eccentric plaques. A 50% reduction in diameter (angiography) in the case of eccentric plaques corresponds to a 50% reduction in area with a PSV ratio of 2, but a 75% reduction in area with a PSV ratio of 4 [25] in the case of concentric plaques, as well as a significantly higher absolute PSV value (Fig. 6); however, this is not taken into account in the stenosis grading studies cited here. Although eccentric plaques are of less hemodynamic relevance, they pose a greater risk for embolism due to their higher plaque density (at the same degree of stenosis), to which shearing forces are applied. This needs to be taken into account when considering re-intervention for recurrent stenosis.
The higher area reduction (hemodynamically higher grade stenosis) in concentric stenosis (75%) compared to eccentric stenosis (50%) at the same diameter reduction (50%). Thus, in the case of a 50% angiographic reduction in diameter, concentric stenosis is clinically (patient symptoms) and hemodynamically (PSV ratio 4) far more relevant than eccentric stenosis (PSV ratio 2). PSV peak systolic velocity
A morphological assessment of in-stent recurrent stenosis, similar to angiography but at a higher spatial resolution due to B‑flow [25] or contrast-enhanced ultrasound (CEUS) [41], can be performed with great accuracy; however, it should be used as an adjunct to duplex ultrasound.
Other stent complications
Other complications following stent implantation include stent dislocation and kinking stenosis distal to the stent in the case of an elongated ICA. In contrast to aortic stent dislocations, for which ultrasound can provide no valid information, the high spatial resolution with high-frequency transducers in carotid stent dislocations visualized in color duplex ultrasound, with flow signals in the stent and between the wall and stent, shows good validity (Fig. 7). Contrast-enhanced ultrasound can reinforce this validity. Using B‑mode/time-motion mode makes it possible to visualize additional pressure-dependent, paradoxical wall movements of the stent against the vascular lumen. Ultrasound is more reliable than angiography in the case of uncoated stents, since angiographic imaging of stent areas that protrude into the lumen, and which contrast medium flows through and around, is virtually impossible.
The Doppler frequency spectrum in the lumen (b) and in the area between the detached stent and vessel wall (a) in stent dislocation: evidence for this is the pulsation in the false lumen in time-motion mode (c, d). In systole (S), the stent (ST) moves from the vessel wall pathway towards the vessel lumen (L) due to the build-up of systolic pressure in the false lumen (x). P plaque
Ultrasound follow-up after PTA and peripheral artery stenting
Background
Endovascular treatment has to some extent replaced bypass surgery for critical ischemia and has become the first-line treatment approach. As in bypass surgery, the patency of revascularization can be impaired by neointimal hyperplasia-related recurrent stenosis, in addition to early stenosis caused by a technical error. Local vascular wall trauma due to angioplasty and stent irritation trigger intimal thickening, which in turn leads to high-grade recurrent stenosis of varying degree or vascular occlusion in the revascularized vessel segment.
The value of ultrasound in follow-up
Despite the risk to revascularization outcome posed by technical errors with intimal flaps or dissections, as well as through recurrent stenosis due to intimal hyperplasia and progressive atherosclerosis, there are only scant studies on the relevance of duplex ultrasound for survival following endovascular revascularization.
Stented peripheral arteries show significantly lower stenotic progression (only 7%) at ultrasound follow-up compared to initial findings following PTA alone (39%) or arterectomy (18%). It is possible that the stent reduces restenosis by elastic plaque-restoring forces or flaps; however, a subsequently higher occlusion rate [42] in the stented group (22%) compared to PTA (6.8%) or arterectomy (8.7%) suggests that the stent triggers a delayed thrombogenic, inflammatory or proliferative response followed by thrombosis. According to Bui et al. 2012 [42], ultrasound follow-up is unnecessary, since in contrast to re-occlusion following bypass surgery, the majority of patients would react with ischemic symptoms in the case of high-grade recurrent stenosis. Furthermore, occlusion of the stented area was foreseeable in only 10% on the basis of high-grade stenosis.
Controversially, two earlier studies [43, 44] already demonstrated the relevance of duplex ultrasound in PTA follow-up and the increased accuracy in predicting recurrent stenosis, particularly in the case of flap-like residual stenosis protruding into the lumen and flow obstruction. Duplex ultrasound was more sensitive here compared to angiography: duplex ultrasound classified 20% of residual stenosis as >50%, whereas angiography classified these as <30%. Stenosis classified as >50% on ultrasound showed significantly reduced patency and a success rate (without high-grade recurrent stenosis or occlusion) of only 11% at 1 year compared to a success rate of 80% if <50% residual stenosis was measured on ultrasound.
Other studies confirmed the importance of duplex ultrasound follow-up. A series of 267 duplex ultrasound examinations in 134 patients following endovascular treatment showed severe stenosis or occlusion in 32.4%. Clinical follow-up and Doppler occlusion pressure alone would have missed 30% of these [45]. Further endovascular correction was necessary in 25% within the 11-month follow-up of this study. Other investigations [46, 47] reported a re-intervention rate of up to 35% in the first year. Another study [48] demonstrated the benefits of ultrasound follow-up after PTA in critical ischemia based on a reduction in the amputation rate: 20% in a group with abnormal ultrasound findings compared to 5% in the group with normal post-interventional ultrasound findings. Early post-interventional ultrasound demonstrated relevant residual stenosis in 56% of cases that were not visualized angiographically.
A limitation that should be mentioned includes the fact that these investigations were retrospective, single-center studies with relatively heterogeneous patient collectives and mixed endovascular treatment forms: PTA, PTA and stent, and arterectomy. The aim of these studies was to evaluate the relevance of follow-up after endovascular treatment and not duplex ultrasound stenosis criteria following PTA and stenting. As such, they worked with the criteria established in duplex ultrasound for native peripheral arteries (PSV > 180 cm/s and PSV ratio >2) as the cut-off for 50% residual or recurrent stenosis (Fig. 8).
External iliac artery (A.I.E) with 50–60% recurrent stenosis in the mid-third of the stent, graded using a peak systolic velocity ratio (PSV ratio) of >2 at a prestenotic PSV of 148 cm/s and an intrastenotic PSV of 341 cm/s. The stent area examined with the transducer to show the spectrum at the same Doppler angle at skin level is marked with arrows (>…<). PSV peak systolic velocity
Stenosis grading following peripheral artery stenting
Compared to the carotid arteries, there are only a handful of studies [49, 50] comparing duplex ultrasound with angiography as the so-called gold standard to find, using ROC curves, an ideal cut-off value of absolute PSV values and a PSV ratio for >50% and higher grade stenosis in peripheral arteries. For example, a threshold velocity of 190 cm/s, with a sensitivity of 88%, specificity of 95%, positive predictive value (PPV) of 98%, negative predictive value (NPV) of 72% and a PSV ratio of 1.5 (93%, 89%, 96%, 81%, respectively) were selected for the femoropopliteal artery in 59 legs examined following PTA for >50% in-stent recurrent stenosis. A PSV of >275 cm/s (sensitivity 97%, specificity 68%, PPV 67% and NPV 97%) and a PSV ratio of >3.5 (sensitivity 74%, specificity 94%, PPV 77% and NPV 88%) was deemed the ideal cut-off value for >80% in-stent stenosis [50]. In another study, the ideal cut-off for >70% stenosis using ROC curves was determined to be 223 cm/s in 143 patients with a sensitivity of 94% and a specificity of 95% [49]. What is striking is that, in contrast to some studies on the stented ICA, the cut-off was set at the level of native stenosis of the femoropopliteal artery and surprisingly somewhat lower in the case of high-grade recurrent stenosis; however, here again, these analyses are, without exception, retrospective with relatively low case numbers and using different endovascular procedures.
The somewhat lower PSV as cut-off in stenosed arteries versus native stenosis could be explained hemodynamically if in-stent stenosis occurred at a more eccentric site. Eccentric stenosis has a smaller area reduction at the same diameter reduction (angiography; also lower clinical relevance due to lower reduction in blood flow) compared to concentric stenosis and thus also a lower PSV (Fig. 5). Furthermore, increasing collateralization in the course of restenosis can lead to reduced blood flow in the stenosed vascular area and thus to reduced PSV at the same degree of stenosis (Fig. 9a–c).
a The collateral function on the prestenotic and intrastenotic peak systolic velocity (PSV) in in-stent stenosis: if collateralization is good (right), flow and thus also prestenotic in-stent PSV (PSV prest) and intrastenotic in-stent PSV (PSV intrast) are lower compared to lower or absent collateralization (left); this leads to lower absolute PSV values at the same degree of stenosis. b The intrastenotic (intrastent.) PSV in the well-collateralized high-grade in-stent stenosis is only 249 cm/s; however, in the case of a prestenotic (presten.) PSV of only 55 cm/s, due to good collateralization (a, c), the PSV ratio is approximately 5 and indicates >80% stenosis according to the continuity law. c Angiography of b with well collateralized in-stent stenosis (arrow). SE stent end, AFS superficial femoral artery, ST stenosis
Systemic factors influencing the absolute PSV value, as well as the stent-induced changes in hemodynamics discussed above, can be circumvented by grading stenosis localized along the course of the stent using the PSV ratio (determined in the stent, Fig. 8; video clips 4 and 5). With the exception of proximal in-stent stenosis in the aortic bifurcation or at the superficial femoral branch, recurrent in-stent stenosis, as in carotid stenosis is therefore most reliably graded using the ratio of intrastenotic PSV/prestenotic PSV according to the continuity law (Fig. 9a–c).
In order to promptly detect technical errors with recurrent stenosis, the first examination in a follow-up program should take place within the first 4 weeks, then at 3–6 months and at 1 year. Patients treated for critical ischemia should be checked every 3 months in the first year and every 6 months thereafter (modified from [51]).
Conclusion
-
Both in vitro and ICA studies showed discrepant PSV measurement results in the grading of in-stent recurrent stenosis (but also of native ICA stenosis) with PSV.
-
In summary, according to the evidence, a PSV of >220 cm/s for >50% and a PSV of >300 cm/s for >70% in-stent recurrent stenosis emerge as threshold velocities following CAS.
-
Increased rigidity of the stented vessel and stent-related lumen reduction are cited as the causes of increased threshold velocity.
-
Since most cases of in-stent recurrent stenosis both in the ICA and peripheral arteries are localized along and in the distal area of the stent, recurrent stenosis can be most accurately graded according to the continuity law using the PSV ratio (intrastenotic PSV/prestenotic PSV in the stent). An in-stent PSV ratio disregards stent-related hemodynamic changes.
References
Lal B, Beach K, Roubin G et al (2012) Restenosis after carotid artery stenting andendarterektomy a secondary analysis of CREST; A randomized controlled trial. Lancet Neurol 11:755–763
Lal BK, Hobson RW, Goldstein J et al (2003) In-stent recurrent stenosis after carotid artery stenting: Life table analysis and clinical relevance. J Vasc Surg 38:1162–1168
Arquizan C, Trinquart L, Touboul P‑J et al (2011) Restenosis is more frequent after carotid stenting than after endarterectomy. Stroke 42:1015–1020
Gröschel K, Riecker A, Schulz JB, Ernemann U, Kastrup A (2005) Systematic review of early recurrent stenosis after carotid angioplasty and stenting. Stroke 36:367–373
Dai Z, Xu G (2017) Restenosis after carotid artery stenting. Vascular 25(6):576–586
AbuRhama AF, Bates M, Stone P, Wulu J (2001) Comparative study of operative treatment and percutaneous transluminal angioplasty/stenting for recurrent carotid disease. J Vasc Surg 34:831–837
Bonati LH, Ederle J, McCabe DJH et al (2009) Long-term risk of carotid restenosis in patients randoly assigned to endovascular treatment or endarterectomy in the Carotid and Vertebral Artery Transluminal Angioplasty Study (CAVATAS): long-term follow-up of a randomised trial. Lancet Neurol 8:908–917
Eckstein H‑H, Ringleb P, Allenberg J‑R et al (2008) Results of the Stent-protected Angioplasty versus Carotid Endarterectomy (SPACE) study to treat symptomatic stenoses after 2 years: A multinational propective, randomised trial. Lancet Neurol 7:893–902
Brott TG, Hobson RW 2nd, Howard G et al (2010) Stenting versus endarterectomy for treatment of carotid-artery stenosis. N Engl Med 363:11–23
Skelly CL, Gallagher K, Fairman RM, Carpenter JP, Velazquez OC, Parmer SS, Woo EY (2006) Risk factors for restenosis after carotid artery angioplasty and stenting. J Vascsurg 44:1010–1015
Willfort-Ehringer A, Ahmadi R, Gessl A, Gschwandtner ME, Haumer A, Lang W et al (2004) Neointimal proliferation within carotid stents is more pronounced in diabetic patients with initial poor glycaemic state. Diabetologia 47:400–406
Zapata-Arriaza E, Moniche F, González A, Bustamante A, Escudero-Martinez I, De la Torre Laviana FJ et al (2016) Predictors of restenosis following carotid angioplasty and stenting. Stroke 47:2144–2147
Cosottini M, Michelassi MC, Bencivelli W, Lazzarotti G, Picchietti S, Orlandi G et al (2010) In stent restenosis predictors after carotid stenting. Stroke Res Treat 2010:864724
Pavela J, Ahanchi S, Steerman SN, Higgins JA, Panneton JM (2014) Grayscale median analysis of primary stenosis and restenosis after carotid endarterectomy. J Vascsurg 59:978–982
Wasser K, Karch A, Gröschel S, Witzenhausen J, Gröschel K, Bähr M et al (2013) Plaque morphology detected with Duplex ultrasound before carotid angioplasty and stenting (CAS) is not a predictor of carotid artery in-stent restenosis, a case control study. Bmc Neurol 13:163
Katano H, Nishikawa Y, Yamada H, Mase M (2017) Calcification in original plaque and restenosis following carotid artery stenting. Surg Neurol Int 8:279
Peterson BG, Longo M, Kibbe MR, Matsumura JS, Blackburn D, Astleford P, Eskandari MK (2005) Duplex ultrasound remains a reliable test even after carotid stenting. Ann Vascsurg 19:793–797
Hakimi M, Knez P, Lippert M, Attigah N, Nelson K, Laub T, Böckler D, Schmitz-Rixen T, Schmandra T (2012) Altered in-stent hemodynamics may cause erroneous upgrding of moderate carotid artery restenosis when evaluated by duplex ultrasound. J Vascsurg 56:1403–1408
AbuRhama AF, Abu-Halimah S, Bensenhaver J, Dean LS, Keiffer T, Emmett M et al (2008) Optimal carotid duplex velocity criteria for defining the severity of carotid in-stent restenosis. J Vasc Surg 48:589–594
Nederkoorn PJ, Brown MM (2009) Optimal cut-off criteria for duplex ultrasound for the diagnosis of restenosis in stented carotid arteries: Review and protocol for a diagnostic study. Bmc Neurol 9:36
Vernhet H, Jean B, Lust S, Laroche JP, Bonafé A, Sénac JP et al (2003) Wall mechanics of stented extracranial carotid artery. Stroke 34(11):e222–4
Kuppler CS, Christie JW, Newton WB III, Ghanami RJ, Craven TE, Berry JL, Hansen KJ (2013) Stent effects on duplex velocity estimates. J Surg Res 183:437–461
Spies C, Doshi R, Spoon J et al (2007) Carotid artery stent type influences duplex ultrasonography derived peak systolic velocity: Findings of an in-vitro model. Catheter Cardiovasc Interv 70:309
Hussain HG, Aparajita R, Khan SZ, Rezayat C, McKinsey JF, Dayal R (2011) Closed-cell stents present with higher velocities on duplex ultrasound compared with open-cell stents after carotid intervention; Short and mid-term results. Ann Vasc Surg 25:55–63
Schäberle W (2018) Ultrasonography in vascular diagnosis 3 edn. Springer, Heidelberg, pp 45, 103–104, 340–343, 324–325
Moneta GL, Edwards JM, Chitwood RW, Taylor LM Jr, Lee RW, Cummings CA et al (1993) Correlation of North American Symptomatic Carotid Endarterectomy Trial (NASCET) angiographic definition of 70 % to 99 % internal carotid artery stenosis with duplex scanning. J Vasc Surg 17:152–157
Arning C, von Reutern GM, Stiegler H, Gortler M (2010) Ultraschallkriterien zur Graduierung von Stenosen der A. carotisinterna – Revision der DEGUM Kriterien und Transfer in NASCETStenosierungsgrade. Ultraschall Med 31:251–257 ((Revision of DEGUM ultrasoundcriteriaforgrading internal carotidarterystenosis and transferto NASCET measurement))
AbuRahma AF, Srivastava M, Stone PA, Mousa AY, Jain A, Dean LS, Keiffer T, Emmett M (2011) Critical appraisal of the Carotid Duplex Consensus criteria in the diagnosis of carotid artery stenosis. J Vasc Surg 53:53–60
Jahromi AS, Ciná CS, Liu Y, Clase CM (2005) Sensitivity and specificity of color duplex ultrasound measurement in the estimation of internal carotid artery stenosis: A systemtic review and meta-analysis. J Vasc Surg 41:962–972
Lal BK, Hobson RW II, Tofighi B, Kapadia I, Cuadra S, Jamil Z (2008) Duplex ultrasound velocity criteria for the stented carotid artery. J Vasc Surg 47:63–73
Stanziale SF, Wholey MH, Boules TN, Selzer F, Makaroun MS (2005) Determining in-stent stenosis of carotid arteries by duplex ultrasound criteria. J Endovasc Ther 12:346–353
Chi YW, White CJ, Woods TC, Goldman CK (2007) Ultrasound velocity criteria for carotid in-stent restenosis. Catheter Cardiovasc Interv 69:349–354
Zhou W, Felkai DD, Evans M, McCoy SA, Lin PH, Kougias P et al (2008) Ultrasound criteria for severe in-stent restenosis following carotid artery stenting. J Vasc Surg 47:74–80
Kwon BJ, Jung C, Sheen SH (2007) CT angiography of stented carotid arteries: Comparison with Doppler ultrasonography. J Endovasc Ther 14:489–497
Chahwan S, Miller MT, Pigott JP, Whalen RC, Jones L, Comerota AJ (2007) Carotid arteria velocity characteristics after carotid artery angioplasty and stenting. J Vasc Surg 45:523–526
Setacci C, Chisci E, Setacci F, Iacoponi F, de Donato G (2008) Grading carotid intrastent restenosis: A 6-year follow-up study. Stroke 39:1189–1196
Cumbie T, Rosero EB, Valentine RJ, Modrall JG, Clagett GP, Timaran CH (2008) Utility and accuracy of duplex ultrasonography in evaluation in-stent restenosis after carotid stenting. Am J Surg 196:623–628
Yan B, Clark M, Kierman T, Schrainfeld R, Lessio S, Rosenfield K (2009) Carotid duplex ultrasound velocity measurements versus intravascular ultrasound in detecting carotid in-stent restenosis. Circ Cardiovasc Interv 2:438–443
Pierce DS, Rosero EB, Modrall JG, Adams-Huet B, Valentine RJ, Clagett GP et al (2009) Open-cell versus closed-cell stent design differences in blood flow velocities after carotid stenting. J Vasc Surg 49:602–606
Lal BK, Kaperonis EA, Cuadra S, Kapadia I, Hobson RW (2007) Patterns of in-stent restenosis after carotid artery stenting: Classification and implications for long-term outcome. J Vasc Surg 46:833–840
Clevert DA, Sommer WH, Helck A, Reiser M (2011) Duplex and contrast enhanced ultrasound (CEUS) in evaluation of in-stent restenosis after carotid stenting. Clin Hemorhreol Microcirc 48(1):199–208
Bui TD, Mills JL, Ihnat DM, Gruessner AC, Goshima KR, Hughes JD (2012) The natural history of duplex-detected stenosis after femoropopliteal endovascular therapy suggests questionable clinical utility of routine duplex surveillance. J Vasc Surg 55:346–352
Kinney EV, Bandyk DF, Mewissen MW, Lanza D, Bergamini TM, Lipchik EO, Seabrook GR, Towne JB (1991) Monitoring funktional patency of percutaneous transluminal anioplasty. Arch Surg 126(6):743–747
Mewissen MW, Kinney EV, Brandyk DF, Reifsnyder T, Seabrook GR, Lipchick EO, Towne JB (1992) The role of duplex scanning versus angiography in predicting outcome after balloon angioplasty in the femoropopliteal artery. J Vasc Surg 15:860–866
Saarinen E, Laukontaus SJ, Albäck A, Venermo M (2014) Duplex surveillance after endovascular revascularisation for critical limb Ischaemia. Eur J Vasc Endovasc Surg 47:418–421
Dick F, Ricco JB, Davies AH, Cao P, Setacci C, de Donato G, Becker F, Robert-Ebadi H, Eckstein HH, De Rango P, Diehm N, Schmidli J, Teraa M, Moll FL, Lepäntalo M, Apelqvist J (2011) Follow-up after revascularisation. Eur J Vasc Endovasc Surg 42:75–90
Giles K, Pomposelli F, Spence T, Hamdan A, Blattman S, Panossian H (2008) Infrapopliteal angioplasty for critical limp ischemia: Relation of transatlantic intersociety consensus class to outcome 176 limbs. J Vasc Surg 48:128–136
Humphries MD, Pevec WC, Laird JR, Yeo KK, Hedayati N, Dawson DL (2011) Early duplex scanning after infrainguinal endovascular therapy. J Vasc Surg 53:353–358
Shrikhande GV, Graham AR, Aparajita R, Gallagher KA, Morrissey NJ, McKinsey JF, Dayal R (2011) Determining criteria for predicting stenosis with ultrasound duplex after endovascular intervention in infrainguinal lesions. Ann Vasc Surg 25:454–460
Baril DT, Rhee RY, Kim J, Makaroun MS, Chaer RA, Marone L (2009) Duplex criteria for determination of in-stent stenosis after angioplasty and stenting of superficial femoral artery. J Vasc Surg 49:133–139
Hodgkiss-Harlow K, Bandyk D (2013) Interpretation of arterial duplex testing of lower-extremity arteries and interventions. Semin Vasc Surg 26:95–104
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
W. Schäberle declares that he has no competing interests.
This article does not contain any studies with human participants or animals performed by any of the authors.
The supplement containing this article is not sponsored by industry.
Additional information
The German version of this article can be found under https://doi.org/10.1007/s00772-018-0433-5.
Caption Electronic Supplementary Material
Video clip 1. Stenosis grading according to the continuity law can be well demonstrated based on recurrent common carotid artery stenosis. Mid-stent luminal narrowing due to restoring forces of a plaque with more than a doubling in PSV along the stent as an indication of >50% stenosis.
Video clip 2. Restoring forces of a plaque cause tapered luminal narrowing at the end of a wall stent following CAS in high-grade ICA stenosis. The PSV ratio (intrastenotic PSV/in stent PSV ICA) of around 2 is a sign of approximately 50% recurrent stenosis. Since blood flows around the end of the stent, it is difficult to angiographically visualize the stent inside.
Video clip 3. A flap-like structure at the distal end of the stent causes a PSV of 250 cm/s. Although, according to the evidence, this PSV is actually an expression of an only 60% diameter reduction, >75% stenosis according to the continuity law is seen and was confirmed angiographically. Prestenotic in-stent 60 cm/s → ratio > 4.
Video clip 4. In-stent recurrent stenosis in the external iliac artery: intrastenotic PSV 348 cm/s; prestenotic in-stent PSV 137 cm/s → ratio 2.5 → 50%–60% recurrent stenosis.
Video clip 5. In-stent recurrent stenosis in the superficial femoral artery: intrastenotic PSV 360 cm/s; prestenotic 82 cm/s → PSV ratio > 4 → recurrent stenosis > 70%. By moving the transducer in parallel at a good Doppler angle, the sudden, stenosis-induced increase in PSV can be visualized.
Rights and permissions
Open Access. This article is distributed under the terms of the Creative Commons Attribution 4.0 International License (http://creativecommons.org/licenses/by/4.0/), which permits unrestricted use, distribution, and reproduction in any medium, provided you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made.
About this article
Cite this article
Schäberle, W. Sonographic grading of recurrent stenosis after carotid stenting and stented peripheral arteries. Gefässchirurgie 24 (Suppl 1), 40–51 (2019). https://doi.org/10.1007/s00772-018-0496-3
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00772-018-0496-3