Abstract
Type B aortic dissection is a relatively rare clinical picture that involves many fields of medicine and requires a multidisciplinary approach. In this report, the disciplines involved constitute the most important aspects of the new S2k guidelines for the diagnosis and treatment of type B aortic dissection. More specifically, the definition and epidemiology, the clinical and technical diagnostic methods, in addition to the imaging methods, are presented, and recommendations for the individual topics based on scientific studies and expert opinion are given. In the guidelines, the various procedures related to the main conservative and surgical treatment regimes in the acute, subacute, and chronic phases of the condition are dealt with.
Zusammenfassung
Die Typ-B-Aortendissektion ist ein relativ seltenes Krankheitsbild, welches in sehr vielen Bereichen der Medizin vorkommt und einen interdisziplinären Behandlungsansatz erfordert. Die beteiligten Disziplinen stellen in diesem Beitrag die wesentlichen Aspekte der neuen S2k-Leitlinie zur Diagnostik und Therapie der Aortendissektion Typ B dar. Im Einzelnen werden die Definition und Epidemiologie, die klinische und instrumentelle Diagnostik sowie die bildgebenden Verfahren dargestellt und zu den einzelnen Themen aufgrund von wissenschaftlichen Studien und Expertenmeinung Empfehlungen gegeben. In der Leitlinie wird außerdem auf die unterschiedlichen Vorgehensweisen in Bezug auf primär konservative und operative Therapieregime in der akuten, subakuten und chronischen Phase der Erkrankung eingegangen.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
A guideline from the German Society for Vascular and Endovascular Surgery (DGG) “Aortic dissection (guideline for the diagnosis and treatment of type B dissection)” was adopted by the board of the German Society of Vascular Surgery on 10 September 2008 and posted on the internet [1].
A guideline review was planned for September 2010 and announced. In collaboration with the Association of the Scientific Medical Societies (AWMF) of Germany and other professional societies, the guideline is now updated, supplemented and published in this article. Based on new study results and expert opinions, the aim of these guidelines is to convey the current state of knowledge on treating type B aortic dissection.
Methods
A search of guideline databases and Medline served as the foundation for the guidelines. A consensus, moderated by the AWMF, was reached by means of nominal group process and the Delphi procedure. The literature search was carried out for specific search terms from April to September 2017, including the databases Medline, Embase, SciSearch, and Cochrane and drew on existing relevant international guidelines. A systematic literature search, where studies are evaluated and classified according to evidence level, was not implemented for these S2k guidelines. They are more a result of a consensus of expert opinions. The guidelines were carried out on behalf of the German Society for Vascular Surgery and Vascular Medicine (DGG) in cooperation with the following medical associations (in alphabetical order):
-
German Society for Anesthesiology and Intensive Care (DGAI)
-
German Society for Angiology, Society for Vascular Medicine (DGA)
-
German Society of Cardiology (DGK)
-
German Society of Internal Medicine (DGIM)
-
German Society of Nephrology (DGfN)
-
German Society of Radiology (DRG)
-
German Society of Surgery (DGCh)
-
German Society for Thoracic and Cardiovascular Surgery (DGTHG)
The consensus process necessary for formulation of recommendations was achieved by the combination of a nominal group process and the Delphi technique. During the nominal group process, participants met for structured meetings directed by a neutral moderator. Consistent consensus was achieved when recommendations and key messages were supported by more than 90% of cast votes.
Type B aortic dissection—definition und classification
Aortic dissection is defined as a tearing apart of the medial layers of the aorta with bleeding within the aortic wall, resulting in separation of the wall layers and subsequently formation of a true and a false lumen. In the majority of patients there is an intimal tear that causes blood to pass between the layers of the media. The adventitia of the false canal can either rupture into the thoracic cavity or a so-called re-entry can lead back into the true lumen through a second distal intimal tear. This results in the typical dissection with a septum between the two lumens. Over time, the false lumen can be partially or completely thrombosed.
Anatomical classification systems were first described by DeBakey et al. in 1965 [2] and by Dailey et al. in 1970 [3]. The DeBakey classification is based on the origin of the intimal tear and the extent of the dissection:
-
DeBakey type I: the dissection originates from the ascending aorta and spreads distally to include the aortic arch or also the descending aorta.
-
DeBakey type II: the dissection originates and is limited to the ascending aorta.
-
DeBakey type III: the dissection originates from the descending aorta and usually spreads distally:
-
Type IIIa: limited to the descending thoracic aorta;
-
Type IIIb: expands to below the diaphragm.
-
According to the Stanford classification, a different distinction is made:
-
Type A: all dissections affecting the ascending aorta, regardless of the entry point location;
-
Type B: all dissections not affecting the ascending aorta. Note that in the Stanford classification involvement of the aortic arch, without involvement of the ascending aorta, belongs to type B.
Note: in recent years a special focus has been placed on the involvement of the aortic arch in primary type B dissection. The term non-A non-B dissection addresses both retrograde dissection after a primary tear in the descending aorta as well as a primary tear in the aortic arch. The value of this characterization lies in the very high probability of the necessity for invasive treatment as soon as the aortic arch is affected.
Based on the time course of the initial event (usually thoracic pain), a distinction is made between acute, subacute and chronic dissection.
-
Acute aortic dissection is defined as the patient presenting within the first 2 weeks after symptom onset or first diagnosis.
-
The subacute phase of aortic dissection is the period between 2 and 6 weeks after symptom onset.
-
The chronic phase of aortic dissection is referred to as after 6 weeks or, according to the guidelines of the European Society of Cardiology [4], when the patient has survived more than 90 days after the acute event.
A further distinction can be made between complicated and uncomplicated aortic dissection. A document from an international expert consensus [5] defined the complicated acute type B aortic dissection as follows:
-
Malperfusion of the aortic branches (spinal, iliac, visceral, renal arteries) indicates impending organ failure and requires early detection. Paraparesis or paraplegia, lower limb ischemia, abdominal pain, nausea and diarrhea are signs of malperfusion. The diagnosis is corroborated by laboratory markers (bilirubin, amylase, liver enzymes, creatinine) and imaging.
-
Refractory hypertension is defined as persisting hypertension despite administration of three different classes of antihypertensive drugs with maximum recommended or maximum tolerated dosage and is evaluated as a sign of instability or renal malperfusion.
-
Increase in periaortic hematoma and hemorrhagic pleural effusion in two consecutive computed tomography (CT) examinations with wait and see medical treatment are indications of impending rupture.
-
Patients with false canal rupture, consecutive circulatory instability, severe hypotension or shock.
Epidemiology and risk factors
A population-based survey in the Oxford region (Oxford Vascular Study, OXVASC; [6]) reported an incidence of aortic dissection in the years 2002–2012 of 6/100,000, whereby Stanford type B dissections were less frequently observed (28.8%) than type A dissections (71.2%). In the population-based Malmö Diet and Cancer Study (MDCS; [7]), type A dissections were also more frequently (58%) seen than type B dissections. In the OXVASC study 67.3% of patients with aortic dissection had arterial hypertension and 61.5% of patients were smokers. Smoking and arterial hypertension were also significant risk factors for developing aortic dissection in the MDCS study, in addition to increasing age, male gender, and low apolipoprotein A-1 levels.
Furthermore, there is a genetic predisposition to aortic dissection. In the International Registry of Aortic Dissection (IRAD; [8]) including 1049 patients with aortic dissection, 53 (5%) with Marfan syndrome were found. Patients with Marfan syndrome were significantly younger, with hypertension and arteriosclerosis being significantly less common than in those without Marfan syndrome. The annual probability of a first-degree relative developing an aortic dissection was 2.77 times higher in the group with a positive family history than in the group with a negative family history. A positive family history is, therefore, the most significant risk factor for aortic dissection.
Clinical picture
In a 17-year analysis of 1476 patients with acute type B aortic dissection [9], the following symptoms and diagnostic findings were observed in the IRAD:
-
Severe or strongest pain ever experienced in 94%;
-
Chest pain in 71%,
-
Back pain in 70%,
-
Syncope in 3%,
-
High arterial blood pressure in 66%,
-
Pulse deficit in 19%,
-
Widened mediastinum in 56–39%.
An inconspicuous chest X‑ray was found in 19–36% of cases, an inconspicuous electrocardiograph (ECG) in 38%. Such findings, therefore, do not exclude a dissection.
Complications
The incidence of malperfusion in aortic dissections is reported to be between 25% and 50% with compression or displacement of the true lumen occurring through dynamic or static mechanisms. For example, the ostium of an aortic side branch can be dynamically displaced by a prolapsing dissection membrane when the membrane intermittently pulsates (systole, diastole), thus obstructing blood flow. If the dissection membrane extends into a side branch and produces a significant stenosis, this is termed static compression. Over the long term, this can lead to vessel thrombosis with permanent ischemia up to end organ infarction.
Dissection-related organ hypoperfusion is one of the major causes of the high mortality and morbidity of aortic dissection patients. On admission the following complications were documented in the IRAD registry [10]:
-
Acute renal failure: 17.9%,
-
Hypotension: 9.7%,
-
Lower limb ischemia: 9.5%,
-
Mesenterial ischemia and/or infarction: 7.4%,
-
Spinal ischemia: 2.5%.
These complications were independent predictors of hospital mortality, with an odds ratio of 9.03 for mesenteric ischemia, 6.43 for hypotension/shock, 3.61 for acute renal failure and 3.02 for lower limb ischemia.
Diagnostics
The personal and family aortic history should be discussed during anamnesis and the clinical evaluation should include a pulse status. The D‑dimer test is highly sensitive at 94% but the specificity is 40–100%. The ECG and chest X‑ray are used to exclude other disorders involving chest pain as a primary symptom, such as myocardial infarction. For a definitive diagnosis, transesophageal echocardiography (TEE), CT and magnetic resonance imaging (MRI) are equally reliable in confirming or ruling out thoracic aortic dissection. The distal portion of the ascending aorta and the aortic arch branches cannot be adequately assessed with TEE. Consequently, CT imaging (MRI to a lesser extent) is the most commonly used diagnostic imaging tool in suspected acute aortic dissection, with the potential addition of TEE.
Treatment
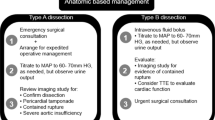
Acute uncomplicated type B aortic dissection
Acute thoracic aortic dissection of the descending aorta should be pharmaceutically treated. All patients suspected of having an acute aortic dissection should be admitted to an acute treatment unit where hemodynamics and urinary output can be reliably monitored. Conservative treatment initially consists of sufficient pain therapy and reduction of the systolic blood pressure to <120 mm Hg or to the lowest level necessary to maintain vital functions. This is done with i.v. administration of beta blockers (esmolol, metoprolol) and vasodilators (labetolol, nitroprusside). A beta blockade should precede the administration of peripheral vasodilators to prevent reflex tachycardia. In the further course, i.v. administration is switched to a corresponding oral antihypertensive medication, lowering the blood pressure to 130/80 mmHg or lower (if tolerated by the patient).
To avoid aortic complications, early thoracic endografting can be selectively considered. The following factors are associated with an increase in aortic diameter [11]:
-
Patient age <60 years,
-
Pulse ≥60 beats/min,
-
Marfan syndrome,
-
Fibrinogen-fibrin cleavage products on admission ≥20 mg/ml,
-
Aortic diameter ≥40 mm at first imaging examination,
-
Patent false lumen,
-
Partially thrombosed false lumen,
-
False lumen in the proximal descending aorta ≥22 mm at initial imaging,
-
Sac formation in the partially thrombosed false lumen,
-
A single entry tear (main entry),
-
False lumen/intima tear located on the inner aortic curvature,
-
Elliptical form of the true lumen/round form of the false lumen,
-
Zones of localized dissection/ulcer,
-
Large entry tear (≥10 mm) localized in the proximal dissection segment,
-
Type B dissection involving the aortic arch.
Acute complicated type B aortic dissection
Endovascular implantation of a thoracic endograft should be the first-line intervention in patients with acute complicated type B aortic dissection. Open surgery is an alternative if thoracic endovascular aortic repair (TEVAR) is contraindicated or has failed. In selected cases, especially if no adequate landing zone is available in zones 1 or 2, debranching of arch vessels or primary implantation of a frozen elephant trunk are possible alternatives. Initial results of the latter technique have shown good outcome [12, 13].
A special endovascular technique to repair dissection is the combination of a stent graft with distal extension by an uncovered dissection stent (PETTICOAT technique [14]). The main entry of the dissection is covered as usual with a proximal stent graft, while the distal metal stent eliminates malperfusion of the distal thoracoabdominal aorta through restored blood flow and aortic remodelling.
Fenestration of the dissection membrane can be carried out as an open or endovascular procedure. In the latter method, an opening between the false and true lumen is created by means of catheter technology and subsequent balloon angioplasty.
Subacute type B aortic dissection
Recommendations for treating subacute aortic dissection mainly refer to the randomized INvestigation of STEnt Grafts in Aortic Dissection (INSTEAD) study [15, 16]. In this study, best medicinal treatment (BMT) (n = 68) was compared with BMT + stent graft (n = 72) in treating uncomplicated type B dissection. Patients had subacute or chronic dissection over a wide range of 2–52 weeks. Initially, the study showed no benefit of endovascular treatment vs. BMT in uncomplicated type B dissections, except for significantly better aortic remodelling. After 5 years, however, the mortality risk from any cause (11.1% vs. 19.3%), the aorta-specific mortality (6.9% vs. 19.3%) and progression of the disease (27.0% vs. 46.1%) were significantly lower for BMT + stent graft than for BMT alone. This suggests that asymptomatic patients should receive endovascular treatment in a timely manner to prevent late complications of the disease.
Chronic type B aortic dissection
Chronic dissection with a maximum thoracic aortic diameter >5.5 cm or a documented increase in the aortic diameter of more than 1 cm within 1 year is an indication for invasive treatment. If malperfusion, aortic rupture or progressive dissection occurs, a chronic aortic dissection should also be treated. The treatment method (endovascular or open) should be selected according to risk factors and anatomical conditions.
Prevention/management of spinal ischemia
Measures to prevent and treat spinal ischemia include [17, 18]:
-
Prevention of hypotensive phases (mean arterial pressure >90 mm Hg),
-
Use of shortest possible coverage of the aorta by the endograft,
-
Carrying out complex endovascular procedures in several stages,
-
Maintaining perfusion of the left subclavian artery, revascularizing if overstenting is planned,
-
Maintaining perfusion of the internal iliac artery,
-
Cerebrospinal fluid (CSF) drainage,
-
Employing local or systemic hypothermia during an open procedure,
-
Optimization of the hemoglobin level,
-
Employing neurophysiological monitoring.
Perioperative CSF drainage effectively reduces the incidence of spinal ischemia as part of open surgical procedures on the thoracic and thoracoabdominal aorta.
There is no comparable evidence regarding CSF drainage in endovascular procedures.
Preventive drainage of CSF should be considered in high-risk patients. Patients scheduled to have extensive thoracic aortic coverage (>200 mm) or having had previous abdominal aortic aneurysm treatment are at high risk for spinal cord ischemia and prophylactic CSF drainage may be considered in cases of endovascular treatment of the thoracic aorta.
Staging procedures can be useful to prevent spinal cord ischemia after TEVAR. The multi-step approach aims to reduce the risk of spinal cord ischemia by preconditioning the spinal cord through development of collateral circulations for spinal perfusion. Endovascular aortic staging procedures include the classical 2‑stage approach, the use of perfusion branches and bridging stents, and segmental artery embolization.
Follow-up
Periodic control in the form of a CT or MRI examination should be performed at least annually after initial treatment of a type B aortic dissection [19, 20]. Regular postprocedural follow-up should be organized by the implanting vascular center. The attending physician should be duly informed and patients should be encouraged to adhere to appointments so that possible complications are detected in a timely manner.
Most important recommendations at a glance
-
In patients with acute severe thoracic pain, the more common acute coronary syndrome should also be considered in addition to aortic dissection.
-
Complications of type B dissections include aortic rupture, organ and limb malperfusion, retrograde type A dissection, refractory arterial hypertension, rapid enlargement of aortic diameter and uncontrollable pain.
-
If acute type B aortic dissection is suspected, chest and abdominal CT angiography should be carried out to evaluate the aorta and first-order aortic branches.
-
All patients suspected of or having a proven acute type B aortic dissection should be referred to a center with expertise in diagnostics (CT, MRI, TEE), conservative intensive care and with surgical and endovascular interventional procedures for aortic dissection.
-
The BMT should always be part of the treatment of patients with acute type B dissection. Patients with acute type B dissection should receive conservative treatment (lowering of blood pressure, administration of analgesics) and intensive monitoring of vital functions should be initiated.
-
To avoid aortic complications in uncomplicated acute type B aortic dissection, early TEVAR may be selectively considered.
-
Endovascular procedures can be performed if clinical risk constellations arise in initially uncomplicated type B dissections, such as poorly controlled pain, poorly controlled arterial hypertension and risk factors for chronic expansion.
-
In patients with complicated acute type B aortic dissection, invasive therapeutic procedures should be used in addition to pharmaceutical treatment.
-
Weighing up the effectiveness and surgical risk, an endovascular intervention is preferable to an open procedure.
-
The endovascular treatment of choice is the implantation of an endovascular aortic prosthesis.
-
Other methods, such as PETTICOAT, endovascular fenestration, the elephant trunk technique, as well as endovascular and open procedures to revascularize specific aortic branches, may be useful in individual cases.
-
When selecting the procedure, individual clinical and anatomical aspects of each patient must be considered.
-
In patients at risk for aortic complications (e.g. only partial false lumen thrombosis, critical false lumen diameter or a large tear at the entry site) and with an appropriate anatomy for an endograft, endovascular treatment for uncomplicated subacute type B aortic dissection should be considered.
-
A maximum thoracic aortic diameter >5.5 cm or a documented increase in the aortic diameter of more than 1 cm within 1 year is an indication for invasive treatment of chronic aortic dissection.
-
Endovascular or open treatment should be selected based on risk factors and anatomical conditions.
-
In cases of malperfusion, aortic rupture or progression, chronic aortic dissection should primarily be treated endovascularly.
-
After initial treatment of a type B aortic dissection, periodic control in the form of a CT or MRI examination should be performed at least annually.
-
Regular postprocedural follow-up should be organized by the implanting vascular center. The attending physician should be duly informed and patients should be encouraged to adhere to appointments so that possible complications are detected in a timely manner.
Literatur
http://www.gefaesschirurgie.de/fileadmin/websites/dgg/download/LL_Aortendissektion_2011.pdf
DeBakey ME, Henley WS, Cooley DA, Morris GC, Crawford ES, Beall AC Jr (1965) Surgical management of dissecting aneurysms of the aorta. J Thorac Cardiovasc Surg 49:130–149
Dailey PO, Trueblood HW, Stinson EB, Wuerflein RD, Shumway NE (1970) Management of acute aortic dissections. Ann Thorac Surg 10:237–247
Erbel R, Aboyans V, Boileau C et al, ESC Committee for Practice Guidelines (2014) ESC Guidelines on the diagnosis and treatment of aortic diseases: Document covering acute and chronic aortic diseases of the thoracic and abdominal aorta of the adult. The Task Force for the Diagnosis and Treatment of Aortic Diseases of the European Society of Cardiology (ESC). Eur Heart J 35:2873–2926
Fattori R, Cao P, De Rango P, Czerny M, Evangelista A, Nienaber C, Rousseau H, Schepens M (2013) Interdisciplinary expert consensus document on management of type B aortic dissection. J Am Coll Cardiol 61:1661–1678
Howard DP, Banerjee A, Fairhead JF, Perkins J, Silver LE, Rothwell PM (2013) Oxford Vascular Study. Population-based study of incidence and outcome of acute aortic dissection and premorbid risk factor control: 10-year results from the Oxford Vascular Study. Circulation 127:2031–2037
Landenhed M, Engström G, Gottsäter A, Caulfield MP, Hedblad B, Newton-Cheh C, Melander O, Smith JG (2015) Risk profiles for aortic dissection and ruptured or surgically treated aneurysms: a prospective cohort study. J Am Heart Assoc 4:e1513
Januzzi JL, Marayati F, Mehta RH, Cooper JV, O’Gara PT, Sechtem U, Bossone E, Evangelista A, Oh JK, Nienaber CA, Eagle KA, Isselbacher EM (2004) Comparison of aortic dissection in patients with and without Marfan’s syndrome (results from the International Registry of Aortic Dissection). Am J Cardiol 94:400–402
Pape LA, Awais M, Woznicki EM et al (2015) Presentation, diagnosis, and outcomes of acute aortic dissection: 17-year trends from the International Registry of Acute Aortic Dissection. J Am Coll Cardiol 66:350–358
Tolenaar JL, Froehlich W, Jonker FH et al (2014) Predicting in-hospital mortality in acute type B aortic dissection: evidence from International Registry of Acute Aortic Dissection. Circulation 130(11Suppl 1):S45–S50
van Bogerijen GH, Tolenaar JL, Rampoldi V, Moll F, van Herwaarden JA, Jonker FH, Eagle KA, Trimarchi S (2014) Predictors of aortic growth in uncomplicated type B aortic dissection. J Vasc Surg 59:1134–1143
Kreibich M, Berger T, Morlock J, Kondov S, Scheumann J, Kari FA, Rylski B, Siepe M, Beyersdorf F, Czerny M (2018) The frozen elephant trunk technique for the treatment of acute complicated Type B aortic dissection. Eur J Cardiothorac Surg 53:525–530
Weiss G, Tsagakis K, Jakob H, Di Bartolomeo R, Pacini D, Barberio G, Mascaro J, Mestres CA, Sioris T, Grabenwoger M (2015) The frozen elephant trunk technique for the treatment of complicated type B aortic dissection with involvement of the aortic arch: multicentre early experience. Eur J Cardiothorac Surg 47:106–114
Nienaber CA, Kische S, Zeller T, Rehders TC, Schneider H, Lorenzen B, Bünger C, Ince H (2006) Provisional extension to induce complete attachment after stent-graft placement in type B aortic dissection: the PETTICOAT concept. J Endovasc Ther 13:738–746
Nienaber CA, Rousseau H, Eggebrecht H, Kische S, Fattori R, Rehders TC, Kundt G, Scheinert D, Czerny M, Kleinfeldt T, Zipfel B, Labrousse L, Ince H (2009) INSTEAD Trial. Randomized comparison of strategies for type B aortic dissection: the INvestigation of STEnt Grafts in Aortic Dissection (INSTEAD) trial. Circulation 120:2519–2528
Nienaber CA, Kische S, Rousseau H, Eggebrecht H, Rehders TC, Kundt G, Glass A, Scheinert D, Czerny M, Kleinfeldt T, Zipfel B, Labrousse L, Fattori R, Ince H (2013) INSTEAD-XL trial. Endovascular repair of type B aortic dissection: long-term results of the randomized investigation of stent grafts in aortic dissection trial. Circ Cardiovasc Interv 6:407–416
Wortmann M, Böckler D, Geisbüsch P (2017) Perioperative cerebrospinal fluid drainage for the prevention of spinal ischemia after endovascular aortic repair. Gefasschirurgie 22(Suppl 2):35–40
Riambau V, Böckler D, Brunkwall J et al (2017) Editor’s choice—management of descending thoracic aorta diseases: clinical practice guidelines of the European Society for Vascular Surgery (ESVS). Eur J Vasc Endovasc Surg 53:4–52
Hahtapornsawan S, Bisdas T, Torsello G, Criado FJ, Austermann M, Donas KP (2016) Importance of early aortic surveillance after endovascular treatment of type B aortic dissection with malperfusion syndrome. Ann Vasc Surg 36:106–111
Hiratzka LF, Bakris GL, Beckman JA et al (2010) ACCF/AHA/AATS/ACR/ASA/SCA/SCAI/SIR/STS/SVM guidelines for the diagnosis and management of patients with thoracic aortic disease: a report of the American College of Cardiology Foundation/American Heart Association Task Force on Practice Guidelines, American Association for Thoracic Surgery, American College of Radiology, American Stroke Association, Society of Cardiovascular Anesthesiologists, Society for Cardiovascular Angiography and Interventions, Society of Interventional Radiology, Society of Thoracic Surgeons, and Society for Vascular Medicine. Circulation 121: e266–369
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
G. Torsello, M. Czerny, R.T. Grundmann, H. Krankenberg, S. Nikol, R. Puls, A. Raddatz, H. Schelzig, R. Schmieder and R. Zahn declare that they have no competing interests.
This article does not contain any studies with human participants or animals performed by any of the authors.
The supplement containing this article is not sponsored by industry.
Rights and permissions
Open Access This article is distributed under the terms of the Creative Commons Attribution 4.0 International License (http://creativecommons.org/licenses/by/4.0/), which permits unrestricted use, distribution, and reproduction in any medium, provided you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made.
About this article
Cite this article
Torsello, G., Czerny, M., Grundmann, R.T. et al. S2k guidelines for the diagnosis and treatment of type B aortic dissection. Gefässchirurgie 24 (Suppl 1), 19–24 (2019). https://doi.org/10.1007/s00772-018-0489-2
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00772-018-0489-2