Abstract
Analytical quality by design (AQbD) is one of the risk-based approach used to develop robust analytical method in compliance with regulatory requirements. The concept of AQbD was recently established in the literature and has proven advantages in the pharma industries. Despite the differential view on AQbD, the International Council for Harmonization (ICH) has released the ICHQ14 document for analytical procedure development. Notably, the enhanced approach of the ICHQ14 document mimics AQbD workflows in analytical procedure development. Among ICHQ14 recommendations, the need for knowledge assessment, multivariate models for proven acceptable range (PARs) as method operable region, sample suitability assessment in robustness, and real-time release testing with product critical quality attribute specifications as the challenging components for pharmaceutical industries. In addition, the integration of ICHQ14 with other ICH documents like ICH Q6A/6B, ICHQ8, ICHQ9, ICHQ10, ICHQ11, and ICHQ12 are well defined in the document. Thus, the revised ICHQ2 (R2) guideline has defined the validation procedure with integration to ICHQ14 documents.







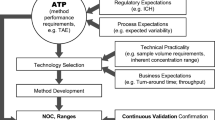
(Source: ICH Q14 Step 2 draft document; p.10 (illustration with little modification)


Similar content being viewed by others
References
Mokhtar HI, Abdel-Salam RA, Hadad GM (2015) Design space calculation by in silico robustness simulation with modeling error propagation in QbD framework of RP-HPLC method development. Chromatographia 78(7–8):457–466. https://doi.org/10.1007/s10337-015-2858-2
Raman NVVSS, Mallu UR, Bapatu HR (2015) Analytical quality by design approach to test method development and validation in drug substance manufacturing. J Chem. https://doi.org/10.1155/2015/435129
ICH Q8 (2009) EMEA/CHMP, 2009, ICH Topic Q 8 (R2) Pharmaceutical Development, Step 5: Note for Guidance on Pharmaceutical Development. Regul. ICH, vol 8, no June
Borman P, Chatfield M, Nethercote P, Thompson D, Truman K (2007) The application of quality by design to analytical methods. Pharm Technol 31:142–152
Hanna-Brown M, Borman P, Bale S, Szucs R, Roberts J, Jones C (2010) Development of chromatographic methods using QbD principles. Sep Sci 2:12–20
Peraman R, Bhadraya K, Reddy YP (2015) “868727,” vol 2015
Deidda R, Orlandini S, Hubert P, Hubert C (2018) Risk-based approach for method development in pharmaceutical quality control context: A critical review. J Pharm Biomed Anal 161:110–121. https://doi.org/10.1016/j.jpba.2018.07.050
Tome T, Žigart N, Časar Z, Obreza A (2019) Development and optimization of liquid chromatography analytical methods by using AQbD principles: Overview and recent advances. Org Process Res Dev 23(9):1784–1802. https://doi.org/10.1021/acs.oprd.9b00238
Prajapati PB, Patel A, Shah SA (2021) Risk and DoE-based DMAIC principle to the multipurpose-RP-HPLC method for synchronous estimation of anti-hypertensive drugs using AQbD approach. J AOAC Int 104(5):1442–1452. https://doi.org/10.1093/jaoacint/qsab079
Urich JAA, Marko V, Boehm K, García RAL, Jeremic D, Paudel A (2021) Development and validation of a stability-indicating uplc method for the determination of hexoprenaline in injectable dosage form using AQbD principles. Molecules. https://doi.org/10.3390/molecules26216597
Hasnain MS, Siddiqui S, Rao S, Mohanty P, Jahan Ara T, Beg S (2016) QbD-driven development and validation of a bioanalytical LC-MS method for quantification of fluoxetine in human plasma. J Chromatogr Sci 54(5):736–743. https://doi.org/10.1093/chromsci/bmv248
Robu S et al (2019) Contribution to the optimization of a gas chromatographic method by QbD approach used for analysis of essential oils from salvia officinalis. Rev Chim 70(6):2015–2020. https://doi.org/10.37358/rc.19.6.7266
Hejmady S, Choudhury D, Pradhan R, Singhvi G, Dubey SK (2021) Analytical quality by design for high-performance thin-layer chromatography method development. In: Handbook of analytical quality by design, pp. 99–113. https://doi.org/10.1016/B978-0-12-820332-3.00007-8.
Almeida J, Bezerra M, Markl D, Berghaus A, Borman P, Schlindwein W (2020) Development and validation of an in-line API quantification method using AQbD principles based on UV-vis spectroscopy to monitor and optimise continuous hot melt extrusion process. Pharmaceutics. https://doi.org/10.3390/pharmaceutics12020150
Weitzel J et al (2021) Understanding quality paradigm shifts in the evolving pharmaceutical landscape: perspectives from the USP quality advisory group. AAPS J 23(6):1–8. https://doi.org/10.1208/s12248-021-00634-5
View of Analytical Quality by Design (AQbD) _ A New Horizon For Robust Analytics in Pharmaceutical Process and Automation.
Jackson P et al (2019) Using the analytical target profile to drive the analytical method lifecycle. Anal Chem. https://doi.org/10.1021/acs.analchem.8b04596
Kochling J, Wu W, Hua Y, Guan Q, Castaneda-Merced J (2016) A platform analytical quality by design (AQbD) approach for multiple UHPLC-UV and UHPLC-MS methods development for protein analysis. J Pharm Biomed Anal 125:130–139. https://doi.org/10.1016/j.jpba.2016.03.031
Borman P et al (2022) Selection of analytical technology and development of analytical procedures using the analytical target profile. https://doi.org/10.1021/acs.analchem.1c03854
Schweitzer M et al (2010) Implications and opportunities of applying QbD principles to analytical measurements. Pharm Technol 34(2):52–59
Nanduri R, “An insight on scientific and risk based approaches for drug product development”.
“‘Use of uncertainty information in compliance assessment,’ in Eurachem/CITAC Guide, 2007.”
Garg LK, Reddy VS, Sait SS, Krishnamurthy T, Vali SJ, Reddy AM (2013) Quality by design: design of experiments approach prior to the validation of a stability-indicating HPLC method for Montelukast. Chromatographia 76(23–24):1697–1706. https://doi.org/10.1007/s10337-013-2509-4
Laures AMF, Wolff JC, Eckers C, Borman PJ, Chatfield MJ (2007) Investigation into the factors affecting accuracy of mass measurements on a time-of-flight mass spectrometer using Design of Experiment. Rapid Commun Mass Spectrom. https://doi.org/10.1002/rcm.2852
Champarnaud E et al (2009) Trace level impurity method development with high-field asymmetric waveform ion mobility spectrometry: systematic study of factors affecting the performance. Rapid Commun Mass Spectrom. https://doi.org/10.1002/rcm.3844
Fukuda IM, Pinto CFF, Moreira CDS, Saviano AM, Lourenço FR (2018) Design of experiments (DoE) applied to pharmaceutical and analytical quality by design (QbD). Braz J Pharm Sci 54(Special Issue):1–16. https://doi.org/10.1590/s2175-97902018000001006
Davis B, Lundsberg L, Cook G (2008) PQLI control strategy model and concepts. J Pharm Innov 3(2):95–104. https://doi.org/10.1007/s12247-008-9035-1
Abhinandana P (2021) IMPLEMENTING OF ANALYTICAL QUALITY BY DESIGN FOR HIGH QUALITY, no. July 2017
Scypinski S, Roberts D, Oates M, Etse J (2002) Pharmaceutical Research and Manufacturers Association acceptable analytical practice for analytical method transfer. Pharm Technol 26(3):84–89
I. 1” “USP <1220> Analytical procedure Lifecycle, USP-NF 2023, “Analytical procedure life cycle,” no. 1 (2022)
Bandopadhyay S, Beg S, Katare OP, Sharma T, Singh B (2020) Integrated analytical quality by design (AQbD) approach for the development and validation of bioanalytical liquid chromatography method for estimation of valsartan. J Chromatogr Sci 58(7):606–621. https://doi.org/10.1093/chromsci/bmaa024
United States Pharmacopeial Convention (2007) VALIDATION OF COMPENDIAL PROCEDURES Test. United States Pharmacopeial Conv., vol 1, p 3445
ICH (2022) ICH—Q14 on analytical procedure development. Int. Conf. Harmon., vol 31, no 0, p 65, [Online]. Available: https://www.ema.europa.eu/en/documents/scientific-guideline/ich-guideline-q14-analytical-procedure-development-step-2b_en.pdf
Alhakeem MA, Ghica MV, Pîrvu CD, Anuța V, Popa L (2019) Analytical quality by design with the lifecycle approach: a modern epitome for analytical method development. Acta Med Marisiensis 65(2):37–44. https://doi.org/10.2478/amma-2019-0010
Kumar N (2020) Analytical method development by using QbD—an emerging approach for robust analytical method development. J Pharm Sci Res 12(10):1298–1305
Kumar N, Sangeetha D (2020) Analytical method development by using QbD-An emerging approach for robust analytical method development. J Pharm Sci Res 12(10):1298–1305
Ahmed S (2018) Technical and regulatory considerations for pharmaceutical product lifecycle: Ich q12. Contract Pharma 4:1–31
Acknowledgements
The authors appreciate international council for harmonization (ICH) and its document ICHQ14 which provided a basis for developing this review and the new illustrations have been drawn for easy understanding of professionals.
Author information
Authors and Affiliations
Contributions
Ramalingam Peraman: Study conception, design, supervision and analysis of manuscript, approved final version of the paper including references Sathuluri Kiranmayi: Collection of data, sequence alignment and drafting of manuscript, editing of manuscript, Ramyasri: Drafting of manuscript Riya Jain: Drafting of manuscript, Dande Aishwarya: alignment and drafting of manuscript, analysis of manuscript, Rahul sir: analysis of manuscript, Edited and approved final version of the paper including references, Ravichandiran: analysis of manuscript, Edited and approved final version of the paper including references.
Corresponding author
Ethics declarations
Conflict of interest
There is no conflict of interest for authors.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Sathuluri, K., Bakam, R., Jain, R. et al. Analytical quality by design (AQbD) in the ICHQ14 guidelines for analytical procedure development. Accred Qual Assur (2024). https://doi.org/10.1007/s00769-024-01587-w
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s00769-024-01587-w