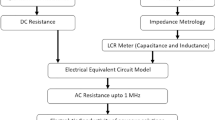
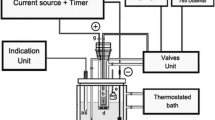
Abstract
The Research Centre for Chemistry—Indonesian Institute of Sciences (RCChem-LIPI) organized an unofficial bilateral comparison on electrolytic conductivity with the Electrochemistry Laboratory—National Institute of Metrology Thailand (NIMT). The electrochemical laboratory RCChem-LIPI, as a Designated Institute for Chemical Metrology in Indonesia, started to develop the secondary method on electrolytic conductivity measurement in 2016. Therefore, the purpose of this unofficial bilateral comparison was to test the ability of RCChem-LIPI laboratory and see the capacity building progress in doing the conductivity measurement. There were two samples containing potassium chloride prepared by RCChem-LIPI, and both samples had the electrolytic conductivity value in the range of 1000 µS cm−1 to 20000 µS cm−1. The samples were sent to NIMT and then measured at the same time duration. The results were compared, analyzed and evaluated based on their assigned values. The assigned values were 15309 µS cm−1 and 6650 µS cm−1 for samples A and B, respectively. The deviation for each measurement result was determined, and the uncertainties were also calculated from the data reported by both laboratories. The data evaluation and analysis confirm that the measurement results for both participant laboratories were in agreement with the assigned value of this unofficial bilateral comparison.




Similar content being viewed by others
References
Ali NS, Mo K, Kim M (2012) A case study on the relationship between conductivity and dissolved solids to evaluate the potential for reuse of reclaimed industrial wastewater. KSCE J Civ Eng 16:708–713
Brinkmann F, Dam NE, Deák E, Durbiano F, Ferrara E, Fükö J, Jensen HD, Máriássy M, Shreiner RH, Spitzer P, Sudmeirer U, Surdu M, Vyskočil L (2003) Primary methods for the measurement of electrolytic conductivity. Accred Qual Assur 8:346–353
Máriássy M, Pratt KW, Spitzer P (2009) Major applications of electrochemical techniques at national metrology institutes. Metrologia 46:199–213
Orrù E (2014) Traceability of electrolytic conductivity measurements for ultra pure water. Politecnico Di Torino, Giugno
Shreiner RH, Pratt KW (2004) Primary standards and standard reference materials for electrolytic conductivity. Natl Inst Stand Technol Spec Publ 260–142:1–31
Asakai T, Maksimov I, Onuma S, Suzuki T, Miura T, Hioki A (2017) New Japanese certified reference materials for electrolytic conductivity measurements. Accred Qual Assur 22:73–81
Breuel U, Werner B, Jehnert D (2009) Metrology in chemistry for pH and electrolytic conductivity traceability dissemination. Chimia 63:643–646
Breuel U, Werner B, Spitzer P, Jensen HD (2016) Experiences with novel secondary conductivity sensors within the German calibration servoce (DKD). NCSLI Meas 3:32–36
Wu YC, Koch WF, Hamer WJ, Kay RL (1987) Review of electrolytic conductance standards. J Solut Chem 16:985–997
Emerson Process Management (2010) Rosemount Analytical, Theory and Application of Conductivity, Application Data Sheet ADS 43-018/rev.D. https://www.emerson.com/documents/automation/application-data-theory-application-of-conductivity-en-68442.pdf. Accessed 5 July 2017
Jameel RH, Wu YC, Pratt KW (2000) Primary standards and standard reference materials for electrolytic conductivity, chapter. US Government Printing Office, Washington
Light TS, Kingman EA, Bevilacqua AC (1995) The conductivity of low concentrations of CO2 dissolved in ultrapure water from 0 to 100 °C. In: 209th American chemical society national meeting; April 2–6, 1995; Anaheim, CA
Guanti RJ, Moran PJ (1986) Measurement of electrolytic conductivity in highly conducting solutions. J Appl Electrochem 16:678–682
BIPM CCQM KCRV WG-19 (2008) Data Evaluation Principles for CCQM Key Comparison. https://www.bipm.org/cc/CCQM/Allowed/15/CCQM09_03.pdf. Accessed 4 June 2017
Acknowledgements
The authors thank to the Research Centre for Chemistry—Indonesian Institute of Sciences (RCChem-LIPI) for financial supporting this study under project “Competency Development Program.”
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that there is no potential conflict of interest.
Rights and permissions
About this article
Cite this article
Krismastuti, F.S.H., Sujarwo, S., Hindayani, A. et al. Competency evaluation on electrolytic conductivity measurement in Indonesia by an unofficial bilateral comparison between RCChem-LIPI and NIMT. Accred Qual Assur 24, 119–125 (2019). https://doi.org/10.1007/s00769-018-1360-6
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00769-018-1360-6