Abstract
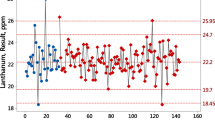
Internal quality control (IQC) is an essential feature of routine analysis, serving to ensure that the uncertainty of results found during the validation of a procedure is maintained over long periods of time. The primary method of IQC is to analyse a surrogate material alongside the test materials in every run of analysis and thus address run-to-run precision (a subset of VIM3-defined ‘intermediate conditions’). This ‘control material’ must be as similar as practicable in composition to the routine test materials, although there are always some differences. Results from the control material (control values) are plotted on a control chart, and out-of-control results have to be investigated and problems rectified. Considerable care is needed in obtaining correct values of the parameters for determining statistical control limits, and these can be adequately estimated only during routine use of the analytical procedure. In contrast, target control limits have to be set on a fitness-for-purpose basis and are necessarily wider that statistical control limits. An additional type of internal quality control can be executed by the analysis of duplicate test portions of some of the actual test samples. This provides a realistic dispersion, but addresses only repeatability precision. A further complication of duplication is that the precision of results typically varies with concentration of the analyte.







Similar content being viewed by others
References
ISO 8258 (1991) Shewhart control charts. International Organisation for Standardisation, Geneva
ISO 7870-1 (2007) Control charts—part 1: general guidelines. International Organisation for Standardisation, Geneva
ISO 7873 (1993) Control charts for arithmetic average and warning limits. International Organisation for Standardisation, Geneva
Thompson M, Wood R (1995) Harmonised guidelines for internal quality control in analytical chemistry laboratories. Pure Appl Chem 67:649–666
Hovind H, Magnussen B, Krysell M, Lund U, Mäkinen I (2011) Internal quality control handbook for chemical laboratories. Nordtest Technical Report 569, 4th edn (Free download from www.nordtest.info)
Thompson M (2012) Anal Meth 4:1598–1611
Danish Ministry of the Environment (2011) Document no 900
Thompson M (2012) J AOAC Int 95:1803–1806
Westgard JO, Barry PL, Hunt MR (1981) Clin Chem 27:493–501
Howarth RJ (1995) Analyst 120:1851–1873
Thompson M, Lowthian PJ (2011) Notes on statistics and data quality for analytical chemists. Imperial College Press, London
Quesenberry CP (1991) J Qual Technol 23:213–224
Codex Alimentarius Commission (2011) Procedural manual. WHO/FAO, Rome, pp 63–78
Fearn T, Fisher S, Thompson M, Ellison SLR (2002) Analyst 127:818–824
Thompson M, Malik KM, Howarth RJ (1998) Anal Comm 35:205–208
Analytical Methods Committee. Sporadic blunders. AMC Technical Briefs no 49
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Thompson, M., Magnusson, B. Methodology in internal quality control of chemical analysis. Accred Qual Assur 18, 271–278 (2013). https://doi.org/10.1007/s00769-013-0955-1
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00769-013-0955-1