Abstract
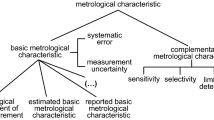
In the ordinary sense the term ”analytical procedure” means a description of what has to be done while performing an analysis without reference to quality of the measurement. A more sound definition of ”analytical procedure” can be given in terms of measurement (quality) assurance, in which a specified procedure to be followed is explicitly associated with an established accuracy of the results produced. The logic and consequences of such an approach are discussed, with background definitions and terminology as a starting point. Close attention is paid to the concept of measurement uncertainty as providing a single-number index of accuracy inherent in the procedure. The appropriateness of the uncertainty-based approach to analytical measurement is stressed in view of specific inaccuracy sources such as sampling and matrix effects. And methods for their evaluation are outlined. The question of a clear criterion for analytical procedure validation is also addressed from the standpoint of the quality requirement which measurement results need to meet as an end-product.
Similar content being viewed by others
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Kadis, R. Analytical procedure in terms of measurement (quality) assurance. Accred Qual Assur 7, 294–298 (2002). https://doi.org/10.1007/s00769-002-0484-9
Issue Date:
DOI: https://doi.org/10.1007/s00769-002-0484-9