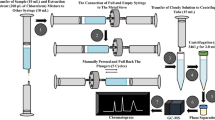
Abstract
In individuals with chronic refractory pouchitis and other gastrointestinal disorders, the combination of ciprofloxacin (CIP) and tinidazole (TIN) is beneficial and safe. As a result, a green micellar high-performance thin-layer chromatographic (HPTLC) approach for the immediate analysis of TIN and CIP in pure, spiked human plasma, and co-formulated tablet dosage form has been developed. It is rapid, extremely easy, sensitive, cost-effective, and environmentally friendly. The stationary phase was Merck aluminum HPTLC plates covered with silica gel 60 F254, while the mobile phase was acetone–ethanol–2% watery sodium dodecyl sulfate (3:4:2, V/V). For quantification of both medications, the densitometric scanner was set at 310 nm. For TIN and CIP, this chromatographic separation yielded symmetric, compact peaks with RF values of (0.22 ± 0.009) and (0.42 ± 0.007), respectively. At 310 nm, the separated spots were densitometrically scanned. For TIN and CIP, the detection thresholds were 6.7 ng/band and 25.03 ng/band, respectively. For TIN and CIP, the quantification limits were 20.3 ng/band and 75.25 ng/band, respectively. The approach was validated according to International Council for Harmonisation (ICH) principles and then used to determine the researched medicines in their various pharmaceutical dosage forms, and human plasma yielding an exceptional percent of recovery. In terms of precision and accuracy, the results were in great accordance with the published approach. This method is suitable for the sequential analysis of the two drugs in pure form, tablet dosage forms, and spiked human plasma due to its simplicity, speed, greenness, robustness, and low cost.
Similar content being viewed by others
1 Introduction
Tinidazole (TIN, Fig. 1) is a chemical compound, 1-[2-(ethylsulfonyl)ethyl]-2-methyl-5-nitro-1H-imidazole, and has a specific antimicrobial activity against anaerobic bacteria. TIN, which was developed in 1972, is one of the most important nitroimidazole antibiotics. TIN is a prodrug that produces a free nitro radical when its nitro group is reduced in trichomonas via a ferredoxin-mediated transport pathway. The antiprotozoal activity of TIN is due to the free nitro radical it generates [1]. TIN is effective against a variety of microorganisms, including Helicobacter pylori, Trichomonas vaginalis, Giardia, and amoebic dysentery [2].
Ciprofloxacin (CIP, Fig. 1), chemically 1-cyclopropyl-6-fluoro-1,4-dihydro-4-oxo-7-(1-piperazinyl)-3-quinolinecarboxylic acid, is a broad-spectrum synthetic fluoroquinolone antibiotic developed by Bayer in 1983 and approved for medicinal use in the United States by the Food and Drug Administration in 1987 [3]. CIP has a great antibiotic action against both Gram-positive and Gram-negative bacteria, like Pseudomonas aeruginosa, Shigella spp., Escherichia coli, and Neisseria [3]. Its antibiotic action is due to its ability to inhibit bacterial DNA gyrase and bacterial DNA topoisomerase, which are essential for bacterial replication [3].
The combination of TIN and CIP is quite useful in the treatment of a variety of bacterial and protozoal diseases. Chronic refractory paucities [4], surgical prophylaxis, surgical wound infections, gynecological infections, prophylaxis in gynecological procedures, respiratory tract infections, ear, nose and throat (ENT) infections, dermatological infections, and intra-abdominal infections were all treated with the mixture [5, 6].
This effective medication combination was separated and quantified using a variety of chromatographic methods, whether in pure form or pharmaceutical dosage forms. Spectrophotometric methods [7,8,9], high-performance liquid chromatography–ultraviolet (HPLC–UV) [10,11,12,13,14], and polarography [15] are among the proposed analytical approaches.
To our knowledge, no high-performance thin-layer chromatographic (HPTLC) approach for the instantaneous analysis of the examined drug combination has been established as of date.
HPTLC techniques, on the other hand, are a promising alternative to common HPLC techniques in some significant analyses, as HPTLC analysis can separate and estimate many analytes in a short time when compared to HPLC, and HPTLC methods use very small sample injection and mobile phase volumes when compared to traditional HPLC analysis, in addition to the possibility of recycling the mobile phase by using it more than once. Because traditional chromatographic procedures still employ huge amounts of non-degradable and ecologically harmful organic solvents, green environmental analysis approaches have become more important in several pharmaceutical research domains.
For these reasons, we developed a green micellar HPTLC method for the simultaneous assessment of the examined drug combination in pure forms and medicinal dosage formulations. HPTLC is an analytical technique that has been utilized to analyze a variety of pharmacological combinations [16,17,18,19,20,21,22] as well as stability-indicating processes [23,24,25].
2 Experimental
2.1 Instrumentation
CAMAG-Korea provided a HPTLC apparatus, which included a semi-automatic sample injection system for sampling under a nitrogen stream, a Hamilton® 100 μL sampling syringe (Bonaduz, Switzerland), and a CAMAG densitometer scanner. HPTLC plates coated with silica gel 60 F254 (Merck, Darmstadt, Germany, 20 × 10 cm and 250 µm thickness) were used as the stationary phase. Acetone, ethanol, and 2% watery sodium dodecyl sulfate (3:4:2, V/V) were used in the development phase. Before sampling, all HPTLC plates were heated to 60 °C for 15 min. The chromatogram progressed to a distance of 9 cm in the TLC chamber (20 × 20 cm) at room temperature using the linear ascending mode; the chamber was thoroughly saturated by the developing phase for 30 min before the linear chromatogram progressed. The chromatogram took roughly 5 min to complete. Finally, the TLC plates were scanned using a CAMAG TLC Scanner 3 densitometric at 310 nm.
2.2 Reagents and materials
Organo Pharma kindly provided authentic TIN (99.9% purity) and CIP (99.8% purity) standards (El Obour industrial zone, Egypt). All the reagents used in the experiment were of analytical grade purity. Acetone, ethanol, ethyl acetate, sodium dodecyl sulfate, and ammonia (25%) were bought from the company El Gomhoria Chemical Co. (Cairo, Egypt).
2.3 Pharmaceutical formulation
Tinifloxacin tablet (batch No. T310421), qualified to contain 500 mg of CIP/tablet, and 600 mg TIN /tablet, was manufactured by Organo Pharma (Obour city, Egypt).
2.4 Preparation of stock standard solutions
Stock standard TIN solutions (100 µg mL–1) were made by dissolving accurately weighted 10 mg of TIN powder in a 100-mL volumetric flask, diluting with roughly 25 mL ethanol, fully dissolved, and then adding ethanol to the mark. Furthermore, stock standard solutions of CIP (100 µg mL–1) were made in the same manner, by properly weighing 10 mg of CIP powder into a 100-mL volumetric flask, diluting with roughly 25 mL ethanol, fully dissolved, and then adding ethanol to the mark.
2.5 Calibration graphs
Various aliquots of the standard stock solutions (1–10 µL of TIN and CIP) were simply spotted on TLC plates to achieve a final drug concentration of 25–1000 ng/band for TIN and 80–1000 ng/band for CIP, respectively. The calibration graphs were created by graphing the area under the peaks versus the drug amount.
2.6 Procedure for pharmaceutical formulation
Ten Tinifloxacin tablets were weighed accurately, finely powdered, and mixed thoroughly. An accurate quantity equivalent to the content of one tablet (600 mg TIN and 500 mg CIP) was weighted and transferred to a 100-mL volumetric flask, and extracted three times with 25 mL ethanol, The contents of the flask were swirled, sonicated for 5 min each time, and filtrated to a 100-mL volumetric flask; then the volume was completed to 100 mL with ethanol to obtain a final solution with concentrations (6 mg mL–1 TIN and 5 mg mL–1 CIP). The prepared solution was spotted on the TLC plates in different volumes to obtain the final substance amount within the calibration range. The general analytical procedure was applied to the tablet extraction.
2.7 Human plasma spiked procedure
According to institutional procedures, plasma samples were collected from normal, healthy, male human volunteers at Zagazig University Hospital (Zagazig, Egypt). An aliquot of 5.0 mL drug-free human plasma sample was transferred to a heparinized tube and vortex-mixed for 2 min at 2000 rpm before centrifugation at 4000 rpm for 30 min. 1.0 mL of drug-free plasma (supernatant) was spiked with 1 mL of stock standard solution into a 10-mL stoppered calibrated tube. Then begins the precipitation of plasma proteins. An aliquot of 2 mL of acetonitrile was added, then completed to 10 mL with distilled water. The tube contents were then centrifuged at 4000 rpm for roughly 15 min. To obtain different solutions within the concentration range of the tested medicines, varied quantities of the resultant supernatant were transferred to a series of 10-mL volumetric flasks and diluted to the mark with ethanol. Following that, the standard analytical method was performed. The drug-free blood sample was treated in the same way as the drug-treated blood sample but without the drug.
3 Results and discussion
3.1 HPTLC method
The therapeutic combination of TIN and CIP has a significant impact and is widely prescribed to treat a variety of diseases caused by bacteria or protozoa such as chronic refractory pouchitis [4]. The suggested HPTLC method proved to be successful in separating the two co-formulated medicines in pure form or in tablet pharmaceutical formulations. For separation and quantification of the two medicines examined, several reagent mixes were used as mobile phases. The separation of the examined pharmaceuticals was achieved using a mobile phase comprising of acetone, ethanol, ethyl acetate, and sodium dodecyl sulfate. Other mobile phases that have been studied have resulted in unsatisfactory separation of the analytes. As a result, we found that a mobile phase made up of acetone, ethanol, and 2% watery sodium dodecyl sulfate (3:4:2, V/V) yielded sharp, symmetric, and non-tailed analytes peaks. The separated medicines showed RF values of (0.22 ± 0.009) and (0.42 ± 0.007) for TIN and CIP, respectively, after being eluted by the selected mobile phase for 5 min (Figs. 2 and 3). For investigation, the densitometric scanner was set to 310 nm. Furthermore, it was discovered that activating the HPTLC plates at 60 °C for 10 min before spotting the sample improved both peak form and repeatability of the suggested approach.
A typical 2D HPTLC calibration chromatogram of A TIN (60 ng/band), B TIN (250 ng/band), C TIN (750 ng/band), D CIP (80 ng/band), E CIP (450 ng/band), F CIP (900 ng/band); laboratory-prepared mixtures of G TIN (85 ng/band), CIP (75 ng/band), H TIN (250 ng/band), CIP (250 ng/band), I TIN (600 ng/band), CIP (500 ng/band), J TIN (250 ng/band), CIP (750 ng/band), K TIN (400 ng/band), CIP (850 ng/band), using acetone–ethanol–2% watery sodium dodecyl sulfate (3:4:2, V/V)
Furthermore, the selected mobile phase comprises a high proportion of sodium dodecyl sulfate, resulting in a micellar mobile phase, which improves the sensitivity of the proposed analytical approach by forming micellar structures with the analytes. Furthermore, because most analysts want to employ environmentally friendly methods in drug analysis, our approach differs from other typical HPTLC methods by using micellar green mobile phase.
3.2 Evaluation of greenness of the HPTLC analytical method
There is now a variety of tools available to assess and compare the greenness of various analytical techniques. In comparison to the prior analytical eco-scale [26], the new green analytical procedure index (GAPI) [26, 27] has the distinct benefit of spanning the whole analytical method. It has five pentagrams, each defining a stage in the analytical approach, such as sample collection and preparation, reagents, and solvents, applied instruments, and the analytical technique’s purpose. GAPI has three color codes, with red indicating a high environmental hazard and yellow and green indicating a lesser hazard and improved greenness. The suggested HPTLC approach was compared to five existing processes that had previously been published. Figure 4 depicts the GAPI which compares the proposed methodology to five previously published analytical methods for the quantification of TIN and CIP. Three of these procedures [12,13,14] employed acetonitrile as an organic solvent, whereas one approach [10] used methanol. As can be seen in Fig. 4a, the suggested technique contains seven green and eight yellow pentagrams, with no red pentagrams in its GAPI. The previously disclosed approaches (b, c, d, e, and f), on the other hand, contain 4, 1, 4, 4, and 5 red pentagrams, respectively, indicating substantial environmental risks. When compared to other previously published chromatographic techniques, the suggested HPTLC method is a green eco-friendly approach, according to the GAPI pentagrams. The main differences from previous procedures was the absence of the extraction step, which is the most inconvenient stage for most analysts, the use of green micellar solvents, the use of lower energy consumption instrumentation, and lower waste production, which can be easily recycled by using it for elution multiple times with multiple TLC plates.
GAPI pictograms for a the proposed method and previously reported methods, b for Ref. [10], c for Ref. [11], d for Ref. [12], e for Ref. [13], and f for Ref. [14]. The red zones represent high ecological impact, yellow zones represents lower impact, and green zones represent safe effect to environment
AGREE [28] is another assessment tool that has been recently introduced on the color code based in GAPI. The main difference from GAPI is that it was based on the twelve green analytical chemistry (GAC) principles. AGREE shows a clock-shaped pictogram, the perimeter of which is divided into twelve sections, each corresponding to a GAC principle. The center of the pictogram shows a numerical value estimating the ecological impact, where the closer to 1, the better is the impact. As shown in Fig. 5, AGREE shows low ecological impact as expressed by the numerical 0.82 value. The perimeter is almost greener, except for the third GAC principle concerned with off-line sampling which is unavoidable as clarified in GAPI pictogram discussion. In the case of using the proposed method for pharmaceutical dosage form analysis, the method would be totally green due to the absence of any required organic solvents. The use of low energy HPTLC equipment, its higher throughput, and simple sample preparation procedures without the need for derivatizing agents account for the better environmentally friendly behavior of the proposed methodology.
3.3 Validation of the proposed HPTLC method
The HPTLC technique was fully verified for accuracy, precision, linearity, limit of quantification (LOQ), limit of detection (LOD), robustness, and selectivity in accordance with the International Council for Harmonisation (ICH) [29].
3.3.1 Linearity and range
The linearity of the proposed HPTLC technique was tested using six concentration points (25, 100, 250, 500, 700, and 1000 ng/spot for TIN and 80, 100, 250, 500, 750, and 1000 ng/spot for CIP), with each concentration being repeated three times and the mean of the three values being determined. The calibration graphs for the substances under investigation were created by graphing the area under the peak vs the relevant drug concentration.
The statistical treatment of the data using regression analysis established the analytical parameters (Table 1).
where A represents the peak area, C represents the drug concentration in ng/band, and r represents the correlation coefficients.
The two formulas, LOD = 3.3σ/S and LOQ = 10σ/S according to ICH guidelines [29, 30], where σ is the standard deviation of the intercept and S is the slope of the calibration graph, are used to determine both LOD and LOQ. LOD values for TIN and CIP were 6.7 and 25.03 ng/band, respectively, while LOQ values for TIN and CIP were 20.3 and 75.34 ng/band, respectively.
3.3.2 Accuracy and precision
Within the analytical range of each of the investigated pharmaceuticals, the accuracy of the developed HPTLC technique was assessed at five concentration levels (150, 250, 400, 500, and 800 ng/band) (triple measurements of each concentration). The percentage of recovery and standard deviation of the measurements are displayed in Table 2. The findings show that the proposed approach is quite accurate.
The precision of the analytical procedure, on the other hand, was assessed by calculating both intra-day and inter-day precision. Three concentration levels of each medication were used to determine intra-day accuracy, and each concentration was reproduced three times on the same day (repeatability). Following development, the inter-day precision of each medication was assessed using three concentration levels; each concentration was repeated three times over three days, and each sample injection volume was repeated three times (intermediate precision). The findings (Table 3) show that the suggested analytical approach has good accuracy at both the inter-day and intra-day levels.
3.3.3 Method selectivity
For approval of the suggested analytical technique’s selectivity, several laboratory-prepared mixtures of the studied pharmaceuticals were analyzed using the proposed analytical technique. The obtained data, given in Table 4, show that the examined medicines had a high percent recovery rate, indicating their selectivity [18, 31, 32]
3.3.4 Method robustness
To test the robustness of the suggested approach, it was utilized to analyses of the researched medicines using the proposed analytical methods but with a modest variation in the mobile phase composition. Any modification in the mobile phase composition results in a modest change in the RF value of the investigated but no change in the area under peak, according to the findings (Table 5).
3.3.5 Application to pharmaceutical formulation
The suggested analytical approach was shown to be effective in analyzing the medicines investigated in their pharmaceutical dosage formulation. The method’s selectivity was tested by looking for any effects from tablet excipients. The results show that tablet excipients did not cause any interference, confirming the accuracy of the suggested approach. The resulting findings were compared to those obtained using the previously described method [10]. In terms of precision and accuracy, Student’s t test and F test are used. Table 6 shows that there was no significant difference in the results produced from both approaches, as demonstrated by Student’s t test and F test, because the calculated values did not exceed the theoretical values at the 95% confidence level. This demonstrates the suggested method’s great precision and accuracy.
3.3.6 Spiked human plasma
With excellent success, the proposed technique was employed to analyze the investigated medicines in spiking human plasma. The regression equation for each drug was used to determine its concentration. The standard solutions of the studied drugs were spiked into human plasma at concentrations of 100, 250, and 500 ng. The results are shown in Table 6. The mean percent of recoveries of the tested drugs concentration in plasma ranged from 98.5 to 99.4, with standard deviations ranging from 0.27 to 0.82, demonstrating that the pharmaceuticals studied may be identified properly and precisely in spiked human plasma without interference.
4 Conclusion
For the simultaneous determination of TIN and CIP, the suggested technique offers a high sensitivity, quick, simple, and green micellar HPTLC method. The suggested approach does not require any prior sample preparation for the extraction phase, which most analysts dislike; also, unlike existing HPLC procedures, the proposed method does not require any expensive chemicals. Because of its greenness and low cost, it is necessary to be utilized in the regular analysis of the examined pharmaceuticals in many quality-control laboratories, in addition to the ability to use the mobile phase several times with multiple plates.
Abbreviations
- TIN:
-
Tinidazole
- CIP:
-
Ciprofloxacin
- HPTLC:
-
High-performance thin-layer chromatography
- GAPI:
-
Green analytical procedure index
References
Choudhary S, Goyal A, Singh GD (2015) Dissolution development of ciprofloxacin and tinidazole in combined tablet dosage form. Int J Pharm Ther 6:33–36
Edwards DI (1993) Nitroimidazole drugs—action and resistance mechanisms II. Mechanisms of resistance J Antimicrob Chemother 31:201–210
Thai T, Salisbury BH, Zito PM (2020) Ciprofloxacin: StatPearls [Internet]. StatPearls Publishing, Treasure Island, FL
Shen B, Fazio VW, Remzi FH, Bennett AE, Lopez R, Brzezinski A, Oikonomou I, Sherman KK, Lashner BA (2007) Combined ciprofloxacin and tinidazole therapy in the treatment of chronic refractory pouchitis. Dis Colon Rectum 50:498–508
Malhotra M, Sharma J, Batra S, Arora R, Sharma S (2003) Ciprofloxacin-tinidazole combination, fluconazole-azithromicin-secnidazole-kit and doxycycline-metronidazole combination therapy in syndromic management of pelvic inflammatory disease: a prospective randomized controlled trial. Indian J Med Sci 57:549–555
Gionchetti P, Calabrese C, Lauri A, Rizzello F (2015) The therapeutic potential of antibiotics and probiotics in the treatment of pouchitis. Expert Rev Gastroenterol Hepatol 9:1175–1181
Bombale M, Kadam S, Dhaneshwar S (1997) Simultaneous spectrophotometric estimation of ciprofloxacin and tinidazole from a combined dosage form. Indian J Pharm Sci 59:265
Gummadi S, Thota D, Varri SV, Vaddi P, Rao VLNS (2012) Development and validation of UV spectroscopic methods for simultaneous estimation of ciprofloxacin and tinidazole in tablet formulation. Int Curr Pharm J 1:317–321
Prathyusha V, Abdul Rahaman S, Revathi S, Renuka G (2013) Development and validation of UV spectrophotometric methods for the simultaneous estimation of ciprofloxacin and tinidazole in tablet dosage form. Int J Pharm Ind Res 3:295–300
Kulsum S, Durga MK, Padmalatha M (2012) A simple and validated RP-HPLC method for the simultaneous estimation of tinidazole and ciprofloxacin in bulk and pharmaceutical dosage forms. Int J Res Dev Pharm Life Sci 2:238–243
Rizk M, Toubar SS, Abou El-Alamin MM, Azab MMM (2014) Micellar high performance liquid chromatographic determination of tinidazole in combination with ciprofloxacin or norfloxacin in bulk, pharmaceutical dosage forms and in spiked human plasma. Eur J Chem 5:439–445
Singh R, Maithani M, Saraf SK, Saraf S, Gupta RC (2009) Simultaneous estimation of ciprofloxacin hydrochloride, ofloxacin, tinidazole and ornidazole by reverse phase–high performance liquid chromatography. Eurasian J Anal Chem 4:161–167
Sirisha T, Gurupadayya B, Sridhar S (2014) Simultaneous determination of ciprofloxacin and tinidazole in tablet dosage form by reverse phase high performance liquid chromatography. Trop J Pharm Res 13:981–987
Ravivarma A, Kumar JS, Reddy S (2013) Development and validation of liquid chromatographic method for the simultaneous estimation of ciprofloxacin and tinidazole in combined dosage form. Int Res J Pharm Appl Sci 3:161–167
Salvi V, Sathe P, Rege P (2011) Simultaneous determination of tinidazole and ciprofloxacin hydrochloride in combined formulation of the two using differential pulse polarography. Anal Chem Indian J 10:308–311
Ibrahim AE, Saraya RE, Saleh H, Elhenawee M (2019) Development and validation of eco-friendly micellar-HPLC and HPTLC–densitometry methods for the simultaneous determination of paritaprevir, ritonavir and ombitasvir in pharmaceutical dosage forms. Heliyon 5:e01518
Saraya RE, Elhenawee M, Saleh H (2018) Development of a highly sensitive high-performance thin-layer chromatography method for the screening and simultaneous determination of sofosbuvir, daclatasvir, and ledipasvir in their pure forms and their different pharmaceutical formulations. J Sep Sci 41:3553–3560
Saraya RE, Elhenawee M, Saleh H (2019) Development of a high-performance thin-layer chromatographic method for the simultaneous determination of newly co-formulated antiviral drugs sofosbuvir and velpatasvir in their pure forms and tablet dosage form. J Planar Chromatogr Mod TLC 32:141–147
Saraya RE, Abdel Salam RA, Hadad GM (2017) High-performance thin-layer chromatography method for the simultaneous determination of itopride, pantoprazole, and mosapride in their formulations and spiked human plasma. J Planar Chromatogr Mod TLC. 30:299–306
Saraya RE, Abdel Hameed EA (2021) Eco-friendly micellar HPTLC technique for the simultaneous analysis of co-formulated antibiotic cefoperazone and sulbactam in pure form and vial pharmaceutical formulation. J Planar Chromatogr Mod TLC 34:121–129
Elhenawee M, Saleh H, Saraya RE (2019) Simultaneous high-performance thin-layer chromatographic determination of ondansetron and pantoprazole in their pure forms and spiked human plasma. J Planar Chromatogr Mod TLC 32:149–156
Saraya RE, Deeb SE, Salman BI, Ibrahim AE (2022) Highly sensitive high-performance thin-layer chromatography method for the simultaneous determination of Molnupiravir, Favipiravir, and Ritonavir in pure forms and pharmaceutical formulations. J Sep Sci 45:2582–2590
Saraya RE, Salam RAA, Hadad GM (2018) Stability-indicating high-performance thin-layer chromatographic determination of ondansetron in pure form and pharmaceutical formulations. J Planar Chromatogr Modern TLC 31:122–128
Kashid AM, Kadam RR (2021) Stability-indicating high-performance thin-layer chromatography method for the simultaneous estimation of emtricitabine and tenofovir alafenamide fumarate. J Planar Chromatogr Mod TLC 34:253–261
Naguib IA, Ali NA, Elroby FA, El Ghobashy MR, Abdallah FF (2020) Validation and eco-scale assessment of stability-indicating HPTLC method for quantitative analysis of carbamazepine and its degradation product, iminostilbene, in pure forms, pharmaceutical preparations, and spiked human plasma. J Planar Chromatogr Mod TLC 33:219–229
Kurowska-Susdorf A, Zwierżdżyński M, Bevanda A, Talić S, Ivanković A, Płotka-Wasylka J (2019) Green analytical chemistry: Social dimension and teaching. Trends Anal Chem 111:185–196. https://doi.org/10.1016/j.trac.2018.10.022
Abdel Hameed EA, Abd El-Naby ZA, El Gindy A, Saraya RE, Al Balawi AN, Zaitone SA, Khairy GM (2022) Two eco-friendly chromatographic methods evaluated by GAPI for simultaneous determination of the fluoroquinolones moxifloxacin, levofloxacin, and gemifloxacin in their pharmaceutical products. Separations 9:330
Pena-Pereira F, Wojnowski W, Tobiszewski M (2020) AGREE—Analytical GREEnness metric approach and software. Anal Chem 92:10076–10082
International Conference on Harmonization (2005) Guideline. Validation of analytical procedures: text and methodology Q2 (R1), Geneva, 11–12
Saraya RE, Salman BI (2022) Sensitive spectrofluorimetric determination of metoclopramide, itopride, mosapride, and trimebutine by fluorescence quenching reaction with eosin Y in pharmaceutical formulations and spiked human plasma. Octahedron Drug Res 1:10–24
Abdel Hameed EA, Abd El-Naby ZA, El Gindy A, Zaitone SA, Alshaman R, Saraya RE, Khairy GM (2022) Two new HPLC methods, assessed by GAPI, for simultaneous determination of four antipsychotics in pharmaceutical formulations: a comparative study. Separations 9:220
Saraya RE, Elhenawee M, Saleh H (2020) Silver nanoparticles synthesis for sensitive spectrophotometric determination of sofosbuvir, lamivudine, and ritonavir in pure forms and pharmaceutical dosage forms. J AOAC Int 103:140–147
Acknowledgements
We gratefully acknowledge the financial support from Organo Pharma (El Obour industrial zone, Egypt) for kindly providing authentic standards.
Funding
Open access funding provided by The Science, Technology & Innovation Funding Authority (STDF) in cooperation with The Egyptian Knowledge Bank (EKB).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Saraya, R.E., Hassan, Y.F., Eltoukhi, W.E. et al. Application of the green analytical procedure index to the simultaneous analysis of co-formulated tinidazole and ciprofloxacin in pure form, tablet dosage form, and human plasma using an environmentally friendly micellar high-performance thin-layer chromatographic technology. JPC-J Planar Chromat 36, 21–30 (2023). https://doi.org/10.1007/s00764-023-00225-4
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00764-023-00225-4