Abstract
Heart failure (HF) has been recognized as a global epidemic with high rates of morbidity, hospitalization, and mortality. The role of amino acids, which provide the body with energy, in the development of HF is still unclear. The aim of this study was to explore changes in serum amino acids in patients with HF and identify potential biomarkers. First, the serum amino acid metabolism profiles of 44 patients with HF and 30 healthy controls (Con) were quantitatively measured. Then, candidate markers were identified through the utilization of T test, multivariate statistical analysis, and receiver operating characteristic (ROC) curve analysis. The results found that there were 11 amino acid levels that were significantly different between patients with HF and Con. Based on ROC curve analysis, the biomarkers of eight amino acids (Glutamic acid, Taurine, l-aspartic acid, l-ornithine, Ethanolamine, l-Serine, l-Sarcosine, and Cysteine) showed high sensitivity and specificity (AUC > 0.90), and binary logistic regression analysis was used in MetaboAnalyst 5.0. Among the amino acids examined, six exhibited notable alterations in accordance with the severity of HF. In conclusion, this study cannot only provide clinicians with an objective diagnostic approach for the early identification of HF, but also enhances comprehension of the underlying mechanisms involved in the pathogenesis of HF.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Heart failure (HF) is a serious condition characterized by fluid retention, dyspnea, and malaise, caused by the inability of cardiac output to meet the metabolic demands of the body's tissues due to damage to the structure or function of the heart, which results in stagnation of the pulmonary and/or somatic circulations as well as reduced perfusion of the organs (Heidenreich et al. 2022). Globally, cardiovascular diseases still lead the mortality rate, higher than tumors and other diseases. Among them, HF is one of the causes of morbidity and mortality, and its incidence continues to rise as human life increases, making it a chronic disease that jeopardizes public health and economic interests (Bozkurt et al. 2021). To date, echocardiography and NT-proBNP are regarded as the "gold standards" for the diagnosis of HF (Feng et al. 2023; Ward et al. 2022). However, echocardiography is characterized by low sensitivity and specificity, long diagnostic time, and technical complexity (Yan et al. 2018), while NT-proBNP is affected by factors such as renal failure (Takase and Dohi 2014; Bachmann et al. 2021), obesity (Bachmann et al. 2021; Parcha et al. 2021), age (Bachmann et al. 2021; Hess et al. 2005; Braisch et al. 2022), and gender (Hess et al. 2005; Braisch et al. 2022; Daubert et al. 2021), which limits its predictive accuracy. Therefore, there is an urgent need to discover new and effective diagnostic and prognostic biomarkers for HF.
Numerous studies have shown that a failing heart is like a "fuel depleted engine", unable to properly use fuel to meet the needs of metabolism (Murashige et al. 2022). To date, most cardiometabolic studies have focused on glucose, fatty acids, and lactic acid, but a growing number of studies have noted alterations in amino acids (Chen et al. 2020; Hakuno et al. 2015). The prognostic value of the branched-chain amino acids (BCAAs)/total amino acid ratio or BCAA/Aromatic amino acids (AAAs) ratio in patients with HF has been reported (Hiraiwa et al. 2020; Kimura et al. 2020). Consequently, we opted to investigate the metabolism of amino acids to identify biomarker patterns that may contribute to an enhanced comprehension of the pathophysiology of HF or facilitate early detection.
The aim of this study was to investigate amino acid metabolism in patients with HF and to analyze the relationship between changes in amino acid metabolism and disease, thereby improving our understanding of the pathogenesis of HF. Consequently, we employed liquid chromatography mass spectrometry (LC–MS/MS) to quantitatively measure the levels of 33 amino acids in the serum of HF patients and healthy controls, aiming to identify significant variations in amino acid profiles among different groups. Our findings help us better understand the development and progression of HF.
Materials and methods
Participants
This study was approved by the Ethics Committee of Beijing Shijitan Hospital Affiliated to Capital Medical University (sjtkyll-1x-2021(21)) in accordance with the 1964 Declaration of Helsinki and its subsequent amendments. Written informed consent was obtained from all participants. Both patients with HF (n = 44) and healthy controls (Con, n = 30) were from the population who came to Beijing Shijitan Hospital affiliated to Capital Medical University during 2021–2023. Serum samples were collected and placed in a coagulant tube, centrifuged (3000 r/min) for 10 min, then supernatant was taken, divided and stored in a refrigerator at – 80 ℃ for later use.
The inclusion criteria for patients with HF were as follows: (1) aged 20–80 years; (2) diagnosed with HF in our hospital according to Framingham criteria; (3) does not have any other clinically diagnosed serious medical condition, including but not limited to tumor, neurological, digestive or mental disorders; or infectious diseases. The sex and age of the healthy control group were matched with the patients with HF, and the level of statistical significance was > 0.05. The inclusion criteria for healthy controls were as follows: (1) aged between 20 and 80 years; (2) no history of self-reported or clinical diagnosis of any chronic serious illness within 3 months, including but not limited to tumor, neurological, kidney, digestive or mental disorders, and infectious diseases; (3) did not receive any treatment within 3 months prior to blood collection.
Quantitative analysis of amino acids
We used the same amino acid detection protocol as in a previously published study (Shi et al. 2023). Briefly, 10 μL of serum sample (or amino acid standard) was added to 10 μL of water and 5 μL of internal standard, mixed appropriately, isopropanol was added to precipitate the protein. The mixture was centrifuged at 12,000 r/min for 10 min. The supernatant (10 μL) was then mixed with 70 μL of borate buffer and 20 μL of AccQ-Tag reagent. After vortexing for 10 s, the mixture was heated at 55 °C for 10 min. We detected amino acids in the samples using UPLC-MS/MS in positive ion multiple reaction monitoring (MRM) mode on a Xevo TQ-XS time-of-flight mass spectrometer (Waters, Shanghai, China). Amino acid abbreviations and mass spectrometric conditions for amino acids and isotopic internal standards are listed in Supplementary Table S1 and S2. Supplementary Table S3 lists the gradient procedure for liquid chromatography.
Statistical analysis
T tests or Wilcoxon tests were performed with SPSS 19.0 statistical software to compare the mean differences between two groups. When conducting multiple tests, apply False Discovery Rate (FDR) adjustment as needed to correct for multiple comparisons. Next, the web-based metabolomics software MetaboAnalyst 5.0 (https://www.metaboanalyst.ca/) was used for analysis. The differentiation between the HF and Con groups was achieved through the utilization of unsupervised Principal Component Analysis (PCA) and Orthogonal Partial Least Squares Discriminant Analysis (OPLS-DA). The variable importance (VIP) was computed for each amino acid projection in the model to demonstrate its contribution to the classification task. Statistical significance was determined by assessing variables with a VIP value exceeding 1.0 and a p value below 0.05. We also performed heat map and receiver operating characteristic curve (ROC) analyses.
Results
Baseline characteristics
The HF patients were classified according to NYHA, including 5 cases in grade II, 17 cases in grade III, and 22 cases in grade IV. In addition, we compared different subgroups of HF patients with the control group. Details of clinical features are presented in Table 1. The box plots of clinical features with significant digits are provided in Supplementary Fig. S1.
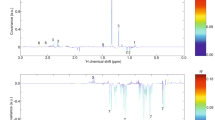
MRM chromatogram of amino acid standards
We measured 33 amino acids, but due to overlapping peaks, we only labeled 20 standard amino acids that constitute proteins in Fig. 1.
Principal component analysis
Principal component analysis (PCA) is used to study the natural distribution of amino acids. Figure 2 shows the PCA plots of the HF group and control group. It can be seen from the PCA diagram that the serum samples are distributed in different regions under different physiological states, indicating that there are differences in the composition and concentration of amino acids. Because clinical samples are affected by many factors, these factors inevitably produce a large number of noisy signals unrelated to information grouping. Therefore, there is a partial overlap in the PCA score plot that cannot be explained. PCA with QC is shown in Supplementary Fig. S2. Standard curve linearity, matrix effects and QCs obtained for the analytes are provided in Supplementary Tables S4 and S5.
Screening and identification of differential amino acids as potential biomarkers of HF
Hierarchical cluster analysis
Heat maps visually represent differences in serum amino acids between patients with HF and controls. To better understand the metabolic changes of the two groups of amino acids, we performed hierarchical cluster analysis on amino acid concentration data obtained from targeted LC–MS analysis. Although some samples overlapped slightly, most samples were clearly divided into two distinct clusters, which is consistent with PCA analysis (Fig. 3).
Orthogonal partial least squares discriminant analysis
Orthogonal partial least squares discriminant analysis (OPLS-DA, Fig. 4A) models showed a clear separation trend between the two groups, revealing more clearly the differences between HF and control. R2Y represents the explanatory rate of the model to the Y matrix, and Q2 represents the predictive power of the model. Our results show that R2Y = 0.85, Q2 = 0.79, both of which are close to 1, indicating that the model is good and the fitting accuracy of the model is high. Figure 4B shows that there are 11 amino acids with VIP scores > 1.0 obtained by the OPLS-DA model. The colored boxes on the right represent the relative concentrations of the corresponding amino acids in the two groups.
OPLS-DA analysis of HF and control (Con) group. A OPLS-DA scores plots; B Top 15 amino acids for VIP score. VIP scores represent the importance of the amino acids involved in the separation in the OPLS-DA model; R2Y represent the percentage of response variance explained by the full model; Q2 represents the predictive performance of the model estimated by cross validation
Classification model to distinguish HF and control
The potential of the 11 amino acids screened before as diagnostic biomarkers for HF was evaluated by ROC curve analysis (Fig. 5). The sensitivity values and area under the ROC curve (AUC) of these amino acids were > 0.7 (Fig. 5A–K). The AUC value close to 1 indicates higher accuracy. Of these, eight amino acids showed high effectiveness (AUC > 0.90) and the other three amino acids showed moderate effectiveness (AUC = 0.7–0.9). In addition, MetaboAnalyst 5.0 was used to perform binary logistic regression for the top eight amino acids (Glutamic acid, Taurine, L-Aspartic acid, L-Ornithine, Ethanolamine, L-Serine, L-Sarcosine, and Cysteine) with the best sensitivity and specificity (AUC = 0.995; Fig. 5L). These results suggest that these eight amino acids could serve as potential biomarkers for early diagnosis or treatment of HF.
Amino acid levels in HF patients stratified by disease severity
To provide valuable insights into the potential variations in amino acid profiles associated with different stages of the disease, the HF patient cohort was divided into three groups based on disease severity for a more detailed analysis of amino acid levels in relation to the severity of HF. Table 2 demonstrates the amino acid levels of the different groups. Notably, six amino acids (Taurine, l-Serine, Ethanolamine, Aspartic acid, Glutamic acid, and Cysteine) demonstrated substantial variations across the severity groups. These findings suggest a potential correlation between disease severity and alterations in amino acid metabolism in HF patients.
Discussion
The metabolic and regulatory functions of amino acids are known to be significant, leading to variations in amino acid levels between patients with different diseases and healthy individuals. Numerous studies have reported alterations in amino acid concentrations in relation to chronic kidney disease (Wang et al. 2019), cardiovascular disease (Li et al. 2017), coronary artery disease and type-2 diabetes mellitus (Magnusson et al. 2013). Therefore, in this study, targeted metabolomics using LC–MS/MS was performed to analyze the differential amino acids between HF patients and healthy controls. We then performed a series of analyses of these differential amino acids, including heat mapping, diagnostic value assessment, and identified several amino acids with high diagnostic value.
Glutamic acid is the most abundant amino acid in the human body and is involved in a variety of physiological functions, such as synthesizing proteins, stimulating immune responses, and regulating cell regulation (Newsholme et al. 2003). Several studies have demonstrated that glutamic acid is among the limited number of amino acids obtained from the heart shortly after coronary artery bypass grafting, and it is the most prevalent amino acid extracted from both the heart and skeletal muscle in such circumstances (Svedjeholm et al. 1991, 1990). Taurine, a semiessential amino acid, highly esteemed for its properties, is the predominant free amino acid found in cardiac myocytes and exhibits beneficial antifibrotic and anti-free radical effects (Ito et al. 2014). Taurine has been reported to reduce cardiomyocyte hypertrophy and fibrosis, and has significant therapeutic effects in chronic HF (Liu et al. 2020). It is currently approved for HF treatment in Japan (Roşca et al. 2022).
Histidine levels decreased and cystine levels increased in patients with HF, which was consistent with literature reports (Kuo et al. 2019; Tessari 2019; Hakuno et al. 2015). Histidine cannot be synthesized in the human body, but it has important physiological functions and is a dietary essential amino acid (Brosnan and Brosnan 2020). First, histidine is a precursor to the synthesis of histamine. Histamine is a bioactive substance that plays a role in regulating vasomotor and blood flow regulation in the cardiovascular system. In patients with HF, histamine may be involved in the regulation of inflammation and cardiovascular function, so histidine, as a precursor of histamine, may be related to the onset and progression of HF (Huang 1985). Second, histidine plays an important role in improving oxidative stress and nitric oxide (NO) metabolism (Yang et al. 2023; Jiang et al. 2016). Oral administration of histidine improves the metabolic pattern in serum and renal tissues of salt-sensitive hypertensive rats, decreases ROS, and increases NO content (Yang et al. 2021). NO has many physiological functions in the cardiovascular system, such as vasodilation and anti-inflammatory effect (McIntyre and Dominiczak 1997). Therefore, histidine metabolism and NO production may play a potential role in the treatment of HF. Finally, histidine has certain antioxidant properties, which can clear free radicals and oxidative stress, reduce myocardial damage and inflammatory response (Megías et al. 2007). Patients with HF are often accompanied by increased oxidative stress, and the antioxidant effect of histidine may help to protect and improve the pathological process of HF (Romuk et al. 2019). Cystine is a sulfur-containing amino acid that plays an important physiological function in human body. The study found that plasma and tissue cystine levels were significantly elevated in patients with HF (Kuo et al. 2019; Tessari 2019; Hakuno et al. 2015). Elevated cystine levels can cause damage to vascular endothelial cells and constriction of blood vessels, which may be associated with the development of HF (Xia et al. 2020).
The stratification of the HF patient cohort based on disease severity has provided valuable insights into the relationship between amino acid profiles and HF progression. Amino acids, as the basic components of protein and peptide synthesis, are essential for normal growth and development of the body. The observed variations in specific amino acids among the severity groups indicate that alterations in amino acid metabolism may be associated with the severity of HF. Inflammatory marker C-reactive protein (CRP) is a common inflammatory cytokine, which plays a key role in the occurrence and development of cardiac dysfunction. With the aggravation of HF, the level of CRP increases. It is worth noting that studies have found that higher levels of taurine are associated with lower levels of CRP, and taurine can significantly reduce the level of inflammatory cytokine CRP, thereby alleviating the damage mediated by inflammatory mediators, and thus may play a myocardial protective effect (Singh et al. 2023; Zhang et al. 2018). Clinical assessment of the severity of HF is often combined with cardiac ultrasound, cardiac MRI and patient symptoms, such as shortness of breath, edema, etc. Amino acids have not been used as indicators to assess the severity of HF, and our study has conducted a preliminary exploration in this respect. These findings warrant further investigation to better understand the underlying mechanisms and potential clinical implications.
Our study highlights plasma amino acid analysis as a potentially useful tool to explore markers for HF risk assessment and prognosis. However, we should acknowledge that the study has some limitations. Most of our results are consistent with previous studies, but there are some inconsistencies. First of all, the small sample size may reduce the statistical power, and this experiment can only be used as a preliminary study, and the accuracy of the eight different amino acids identified as potential biomarkers needs more research to verify. Second, the metabolism of amino acids in the body is relatively complex, and the changes of amino acid metabolites and related specific metabolic pathways will be focused on in the following studies. On the other hand, due to the nature of single-center case–control studies, larger studies are needed to answer other questions before the identified amino acids can be used in clinical decision-making, such as whether amino acid-directed therapy can improve treatment outcomes.
Conclusion
In this study, the levels of 11 amino acids were differentially regulated in the serum of patients with HF, while further validation showed that 8 amino acids may be most associated with the disease state. This extends our understanding of the pathophysiology of the disease. Future studies could identify appropriate biomarkers for early detection to help physicians diagnose HF early and guide treatment and prognosis.
Availability of data and materials
Data sets used and/or analyzed in this study are available on request from the corresponding authors.
References
Bachmann KN, Gupta DK, Xu M, Brittain E, Farber-Eger E et al (2021) Unexpectedly low natriuretic peptide levels in patients with heart failure. JACC Heart Fail 9(3):192–200. https://doi.org/10.1016/j.jchf.2020.10.008
Bozkurt B, Fonarow GC, Goldberg LR, Guglin M, Josephson RA, Forman DE et al (2021) Cardiac rehabilitation for patients with heart failure: JACC expert panel. J Am Coll Cardiol 77(11):1454–1469. https://doi.org/10.1016/j.jacc.2021.01.030
Braisch U, Koenig W, Rothenbacher D, Denkinger M, Friedrich N, Felix SB et al (2022) N-terminal pro brain natriuretic peptide reference values in community-dwelling older adults. ESC Heart Fail 9(3):1703–1712. https://doi.org/10.1002/ehf2.13834
Brosnan ME, Brosnan JT (2020) Histidine metabolism and function. J Nutr 150(Suppl 1):2570S-2575S. https://doi.org/10.1093/jn/nxaa079
Chen WS, Wang CH, Cheng CW, Liu MH, Chu CM, Wu HP et al (2020) Elevated plasma phenylalanine predicts mortality in critical patients with heart failure. ESC Heart Fail 7(5):2884–2893. https://doi.org/10.1002/ehf2.12896
Daubert MA, Yow E, Barnhart HX, Piña IL, Ahmad T, Leifer E et al (2021) Differences in NT-proBNP response and prognosis in Men and Women with heart failure with reduced ejection fraction. J Am Heart Assoc 10(10):e019712. https://doi.org/10.1161/JAHA.120.019712
Feng S, Wu X, Bao A, Lin G, Sun P, Cen H et al (2023) Machine learning-aided detection of heart failure (LVEF ≤ 49%) by using ballistocardiography and respiratory effort signals. Front Physiol 13:1068824. https://doi.org/10.3389/fphys.2022.1068824
Hakuno D, Hamba Y, Toya T, Adachi T (2015) Plasma amino acid profiling identifies specific amino acid associations with cardiovascular function in patients with systolic heart failure. PLoS ONE 10(2):e0117325. https://doi.org/10.1371/journal.pone.0117325
Heidenreich PA, Bozkurt B, Aguilar D, Allen LA, Byun JJ, Colvin MM et al (2022) AHA/ACC/HFSA guideline for the management of heart failure: executive summary: a report of the American college of cardiology/American heart association joint committee on clinical practice guidelines. Circulation 145(18):e876–e894. https://doi.org/10.1161/CIR.0000000000001062
Hess G, Runkel S, Zdunek D, Hitzler WE (2005) Reference interval determination for N-terminal-B-type natriuretic peptide (NT-proBNP): a study in blood donors. Clin Chim Acta 360(1–2):187–193. https://doi.org/10.1016/j.cccn.2005.04.031
Hiraiwa H, Okumura T, Kondo T, Kato T, Kazama S, Ishihara T et al (2020) Usefulness of the plasma branched-chain amino acid/aromatic amino acid ratio for predicting future cardiac events in patients with heart failure. J Cardiol 75(6):689–696. https://doi.org/10.1016/j.jjcc.2019.12.016
Huang WQ, Yu ZM (1985) Role of histamine in cardiovascular regulation and its mechanism. J Suzhou Med College 02:73–75
Ito T, Schaffer S, Azuma J (2014) The effect of taurine on chronic heart failure: actions of taurine against catecholamine and angiotensin II. Amino Acids 46(1):111–119. https://doi.org/10.1007/s00726-013-1507-z
Jiang WD, Qu B, Feng L, Jiang J, Kuang SY, Wu P et al (2016) Histidine prevents Cu-induced oxidative stress and the associated decreases in mRNA from encoding tight junction proteins in the intestine of grass carp (Ctenopharyngodon idella). PLoS ONE 11(6):e0157001. https://doi.org/10.1371/journal.pone.0157001
Kimura Y, Okumura T, Kazama S, Shibata N, Oishi H, Arao Y et al (2020) Usefulness of plasma branched-chain amino acid analysis in predicting outcomes of patients with nonischemic dilated cardiomyopathy. Int Heart J 61(4):739–747. https://doi.org/10.1536/ihj.20-010
Kuo WK, Liu YC, Chu CM, Hua CC, Huang CY, Liu MH et al (2019) Amino acid-based metabolic indexes identify patients with chronic obstructive pulmonary disease and further discriminates patients in advanced BODE stages. Int J Chron Obstr Pulm Dis 14:2257–2266. https://doi.org/10.2147/COPD.S220557
Li T, Zhang Z, Kolwicz SC Jr, Abell L, Roe ND, Kim M et al (2017) Defective branched-chain amino acid catabolism disrupts glucose metabolism and sensitizes the heart to ischemia-reperfusion injury. Cell Metab 25(2):374–385. https://doi.org/10.1016/j.cmet.2016.11.005
Liu J, Ai Y, Niu X, Shang F, Li Z, Liu H et al (2020) Taurine protects against cardiac dysfunction induced by pressure overload through SIRT1-p53 activation. Chem Biol Interact 317:108972. https://doi.org/10.1016/j.cbi.2020.108972
Magnusson M, Lewis GD, Ericson U, Orho-Melander M, Hedblad B, Engström G et al (2013) A diabetes-predictive amino acid score and future cardiovascular disease. Eur Heart J 34(26):1982–1989. https://doi.org/10.1093/eurheartj/ehs424
McIntyre M, Dominiczak AF (1997) Nitric oxide and cardiovascular disease. Postgrad Med J 73(864):630–634. https://doi.org/10.1136/pgmj.73.864.630
Megías C, Pedroche J, Yust MM, Girón-Calle J, Alaiz M, Millan F et al (2007) Affinity purification of copper chelating peptides from chickpea protein hydrolysates. J Agric Food Chem 55(10):3949–3954. https://doi.org/10.1021/jf063401s
Murashige D, Jung JW, Neinast MD, Levin MG, Chu Q, Lambert JP et al (2022) Extra-cardiac BCAA catabolism lowers blood pressure and protects from heart failure. Cell Metab 34(11):1749-1764.e7. https://doi.org/10.1016/j.cmet.2022.09.008
Newsholme P, Procopio J, Lima MM, Pithon-Curi TC, Curi R (2003) Glutamine and glutamate—their central role in cell metabolism and function. Cell Biochem Funct 21(1):1–9. https://doi.org/10.1002/cbf.1003
Parcha V, Patel N, Kalra R, Suri SS, Arora G, Wang TJ et al (2021) Obesity and serial NT-proBNP levels in guided medical therapy for heart failure with reduced ejection fraction: insights from the guide-it trial. J Am Heart Assoc 10(7):e018689. https://doi.org/10.1161/JAHA.120.018689
Romuk E, Wojciechowska C, Jacheć W, Nowak J, Niedziela J, Malinowska-Borowska J et al (2019) Comparison of oxidative stress parameters in heart failure patients depending on ischaemic or nonischaemic aetiology. Oxid Med Cell Longev 2019:7156038. https://doi.org/10.1155/2019/7156038
Roşca AE, Vlădăreanu AM, Mirica R, Anghel-Timaru CM, Mititelu A, Popescu BO et al (2022) Taurine and its derivatives: analysis of the inhibitory effect on platelet function and their antithrombotic potential. J Clin Med 11(3):666. https://doi.org/10.3390/jcm11030666
Shi Z, Yang C, Xu X, Wu W, Jiang D, Yan D (2023) Plasma metabolite profiles identify pediatric medulloblastoma and other brain cancer. Anal Bioanal Chem 415(3):471–480. https://doi.org/10.1007/s00216-022-04427-3
Singh P, Gollapalli K, Mangiola S, Schranner D, Yusuf MA, Chamoli M et al (2023) Taurine deficiency as a driver of aging. Science 380(6649):eabn9257. https://doi.org/10.1126/science.abn9257
Svedjeholm R, Svensson S, Ekroth R, Milocco I, Nilsson F, Vinnars E et al (1990) Trauma metabolism and the heart: studies of heart and leg amino acid flux after cardiac surgery. Thorac Cardiovasc Surg 38(1):1–5. https://doi.org/10.1055/s-2007-1013981
Svedjeholm R, Ekroth R, Joachimsson PO, Ronquist G, Svensson S, Tydén H (1991) Myocardial uptake of amino acids and other substrates in relation to myocardial oxygen consumption four hours after cardiac operations. J Thorac Cardiovasc Surg 101(4):688–694
Takase H, Dohi Y (2014) Kidney function crucially affects B-type natriuretic peptide (BNP), N-terminal proBNP and their relationship. Eur J Clin Invest 44(3):303–308. https://doi.org/10.1111/eci.12234
Tessari P (2019) Nonessential amino acid usage for protein replenishment in humans: a method of estimation. Am J Clin Nutr 110(2):255–264. https://doi.org/10.1093/ajcn/nqz039
Wang YN, Ma SX, Chen YY, Chen L, Liu BL, Liu QQ et al (2019) Chronic kidney disease: biomarker diagnosis to therapeutic targets. Clin Chim Acta 499:54–63. https://doi.org/10.1016/j.cca.2019.08.030
Ward Z, Schmeier S, Pearson J, Cameron VA, Frampton CM, Troughton RW et al (2022) Identifying candidate circulating RNA markers for coronary artery disease by deep RNA-sequencing in human plasma. Cells 11(20):3191. https://doi.org/10.3390/cells11203191
Xia H, Li Z, Sharp TE 3rd, Polhemus DJ, Carnal J, Moles KH et al (2020) Endothelial cell cystathionine γ-lyase expression level modulates exercise capacity, vascular function, and myocardial ischemia reperfusion injury. J Am Heart Assoc 9(19):e017544. https://doi.org/10.1161/JAHA.120.017544
Yan L, Li J, Wu Q, Chen L (2018) Specific miRNA expression profile in the blood serum of cardiac myxoma patients. Oncol Lett 16(4):4235–4242. https://doi.org/10.3892/ol.2018.9209
Yang P, Deng F, Yuan M, Chen M, Zeng L, Ouyang Y et al (2023) Metabolomics reveals the defense mechanism of histidine supplementation on high-salt exposure-induced hepatic oxidative stress. Life Sci 314:121355. https://doi.org/10.1016/j.lfs.2022.121355
Yang PF, Zhao XR, Zhou LX, Jin YX, Zheng XW, Ouyang YN et al (2021) Protective effect of oral histidine on hypertension in Dahl salt-sensitive rats induced by high-salt diet. Life Sci 270:119134. https://doi.org/10.1016/j.lfs.2021.119134
Zhang ZL, Liu X, Hu J, Du SD, Ren FY (2018) Molecular mechanism of taurine metabolism and protection in mice with myocardial ischemia. Chin J Gerontol 38(16):3996–3999. https://doi.org/10.3969/j.issn.1005-9202.2018.16.061
Acknowledgements
This study was generously funded by the National Key Research and Development Program of China (No. 2020YFF01014606) and Beijing Shijitan Hospital Affiliated to Capital Medical University (No. 2021-C03).
Funding
National Key Research and Development Program of China, 2020YFF01014606, Beijing Shijitan Hospital Affiliated to Capital Medical University, No. 2021-C03.
Author information
Authors and Affiliations
Contributions
DJ and LY conceived and designed the framework of this study.LB and ZS collected, analyzed, and organized the data. XX performed the statistical analysis. CY drafted the manuscript.
Corresponding author
Ethics declarations
Ethical approval
This study was approved by the Ethics Committee of Beijing Shijitan Hospital Affiliated to Capital Medical University (sjtkyll-1x-2021(21)) in accordance with the 1964 Declaration of Helsinki and its subsequent amendments. Written informed consent was obtained from all participants.
Conflict of interest
The author states that there is no conflict of interest.
Additional information
Handling editor: D. Tsikas.
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Yang, C., Shi, Z., Bao, L. et al. Targeted metabolomic analysis of serum amino acids in heart failure patients. Amino Acids 56, 22 (2024). https://doi.org/10.1007/s00726-024-03385-7
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s00726-024-03385-7