Abstract
Histamine is a biogenic amine implicated in various biological and pathological processes. Convenient cellular models are needed to screen and develop new antihistamine agents. This report aimed to characterize the response of neurons differentiated from mouse P19 embryonal carcinoma cells to histamine treatment, and to investigate the modulation of this response by antihistamine drugs, vegetal diamine oxidase, and catalase. The exposure of P19 neurons to histamine reduced cell viability to 65% maximally. This effect involves specific histamine receptors, since it was prevented by treatment with desloratadine and cimetidine, respectively, H1 and H2 antagonists, but not by the H3 antagonist ciproxifan. RT-PCR analysis showed that P19 neurons express H1 and H2 receptors, and the H3 receptor, although it seemed not involved in the histamine effect on these cells. The H4 receptor was not expressed. H1 and H2 antagonists as well as vegetal diamine oxidase diminished the intracellular Ca2+ mobilization triggered by histamine. The treatment with vegetal diamine oxidase or catalase protected against mortality and a significant reduction of H2O2 level, generated from the cells under the histamine action, was found upon treatments with desloratadine, cimetidine, vegetal diamine oxidase, or catalase. Overall, the results indicate the expression of functional histamine receptors and open the possibility of using P19 neurons as model system to study the roles of histamine and related drugs in neuronal pathogenesis. This model is less expensive to operate and can be easily implemented by current laboratories of analysis and by Contract Research Organizations.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Histamine is involved in various physiological and pathological processes. This biogenic amine stimulates gastric secretion and modulates the immune system activating inflammatory processes (Rocha et al. 2014; Branco et al. 2018). The excess of exogenous histamine can generate pseudo-allergies, whereas endogenous excess is associated with allergic phenomena and anaphylaxis (Kemp and Lockey 2002; Maintz and Novak 2007). Histamine modulates heart rate and contractility, vasodilatation, and paracellular permeability (Obara et al. 2020). It acts on neurons of the enteric nervous system (ENS) with effects on smooth muscle and intestinal contraction (Spencer and Hu 2020), and as a neuromodulator/neurotransmitter in the central nervous system (CNS). Histamine is involved in the sleep waking cycles, synaptic plasticity, learning, and in neurological disorders, such as migraine, neuroinflammation, epilepsy, and brain infarction (Haas et al. 2008; Yuan and Silberstein 2018), indicating the importance of drug development targeting the brain histamine system.
In the CNS, histamine synthesized by the enzymatic decarboxylation of L-histidine is stored in synaptic vesicles via vesicular monoamine transporter 2 in histaminergic neurons. Upon stimulation, histamine is released to extra neuronal spaces and exerts its effects through interactions with histamine receptors (H-receptors) (Brown et al. 2001; Duan and Wang 2010; Huang et al. 2018). Four histamine receptors (H1–H4) have been identified. In general, H1, H2, and H3 are distributed in various tissues, including the brain and the ENS, while H4 is found mostly on immune and intestinal cells. In the CNS, H1, and H2 are postsynaptic receptors present on neurons. Their activation by micromolar histamine levels excites neurons or potentiates excitatory inputs (Panula. 2021). Effects driven by histamine may interfere with cellular survival. Activation of H1 and H2 receptors in CNS neurons causes the release of calcium ions (Ca2+) from the endoplasmic reticulum followed by the production of arachidonic acid, nitric oxide (NO), and reactive oxygen species (ROS) (Molina-Hernández and Velasco 2008; Shahid et al. 2009; Panula et al. 2015). The production of ROS is controlled by defense systems which can involve superoxide dismutase, catalase (CAT), glutathione peroxidase, and glutathione reductase (Halliwell. 2007; Flora. 2009). H3 is a pre- and postsynaptic receptor mainly present on astrocytes and histaminergic neurons (Tiligada et al. 2011; Panula et al. 2015; Panula. 2021). Its activation by nanomolar histamine regulates the release and synthesis of this biogenic amine (Schwartz et al. 1991; Leurs et al. 2012). In these cells, histamine is internalized via organic cation transporter 3 (OCT3) and via plasma membrane monoamine transporters (PMAT) and finally metabolised by histamine N-methyltransferase (Duan and Wang 2010; Yoshikawa et al. 2019).
Antihistamine agents targeting H-receptors, such as desloratadine (DES), cimetidine (CIM), or ciproxifan (CPX), have been developed to alleviate histamine-induced dysfunctions (Leurs et al. 2005; Canonica and Blaiss 2011; Jafarzadeh et al. 2019). The spectrum of histamine functions in the CNS has been greatly expanded during the last decades. However, much remains to be learned about H-receptors with respect to the development of new and improved antihistamine agents for histamine dysfunctions that cause pathophysiological processes in neurons. The use of convenient cellular models in preclinical studies is suitable for the development of new drugs. P19 embryonal carcinoma cells have been used extensively to generate the P19 neuronal model due to the ability of these cells to differentiate into neurons in culture (McBurney et al. 1988). These P19 neurons can mimic those of the CNS based on the spectra of neurotransmitters and neuropeptides they produce (Staines et al. 1994; Ulrich and Majumder 2006). Previous works mentioned functional H1 receptors on undifferentiated P19 embryonal carcinoma cells (Bloemers et al. 1993), whereas our report is on P19 differentiated neurons. More recently, Molina-Hernández and Velasco (2008) have used E14 embryos extracted from pregnant Wistar rats to obtain multipotent neural stem cells. This is a longer and probably more expensive method. The advantage of cultured P19 differentiated neurons for serial drug evaluation of antihistamine drugs is that the whole procedure is shorter, easier to operate and avoids regulatory limitations for the use and handling of animals. Since the ENS contains a diversity of neurons, it is not excluded that P19 neurons could further serve as a model for the investigations of this system embedded in the lining of the gastrointestinal tract, which contains many of the neurotransmitters and neuropeptides found in the CNS (Furness. 2000; Mittal et al. 2017; Kulkarni et al. 2018; Spencer and Hu 2020).
Considering that neurons in the CNS and the ENS express H1, H2, and H3 receptors (Breunig et al. 2007), it was of interest to investigate to which extent P19 neurons can serve as a model to evaluate the effect of histamine and of antihistamine agents involved in the treatment of histamine-induced dysfunctions. Also, as histamine antagonists may have undesirable side effects, vegetal diamine oxidase (vDAO), such as that from Fabaceae, was suggested as an alternative treatment to alleviate various histamine-related dysfunctions associated with allergic conditions (Mondovi et al. 2013) and intestinal disorders (Neree et al. 2020). This enzyme (EC 1.4.3.22), in fact, can catalyze the oxidative deamination of histamine to the corresponding aldehyde, with the production of H2O2 and NH3. It also reduces neutrophil activation (Pietrangeli et al. 2020) and the consequent cellular oxidative damage (Masini et al. 2007). The aim of this work was thus to characterize the response to histamine of cultured P19 neurons and the modulation of this response by three common antihistamine agents (DES, CIM, and CPX) as well as by vDAO or CAT.
Materials and methods
vDAO purification and characterization
The vDAO was purified from Lathyrus sativus seedlings as previously reported (Blemur et al. 2016), but samples were homogenized in 50 mM phosphate buffer (pH 5.5) containing 200 mM NaCl. The vDAO samples were lyophilized and stored at −80° C until characterization. They contained 0.35 ± 0.05 mg protein/mg solid, as determined by the Bio-Rad assay with bovine serum albumin (BSA) as the standard. The vDAO-specific activity of 19 0.0 ± 2.6 U/mg solid was measured as reported (Jumarie et al. 2017). One enzyme unit was the amount of DAO enzyme able to catalyze the oxidation of 1 µmol of putrescine substrate per minute.
Cell culture
Murine P19 embryonal carcinoma cells (1 × 106) were cultured in 100 mM tissue-grade Petri dishes (Fisher Scientific, Montreal, QC, Canada) containing 10 mL complete alpha-Modified Eagle's Essential Medium (α-MEM; Invitrogen, Burlington, ON, Canada) supplemented with 10% (v/v) fetal bovine serum (FBS; Wisent Inc., St-Bruno, QC, Canada), 50 U/mL penicillin, and 50 µg/mL streptomycin (Sigma-Aldrich, Oakville, ON, Canada) at 37° C in a 5% CO2-95% humidified air atmosphere. Cells were passaged every 2 days by trypsinization (0.025% v/v trypsin-1 mM EDTA; Sigma-Aldrich, Oakville, ON, Canada) (Ducharme et al. 2010). The P19 cell line was obtained from Dr. M. W. McBurney (Université d'Ottawa, Ottawa, ON, Canada).
Neuronal differentiation was carried out by culturing Pl9 cells for 4 days in the presence of retinoic acid (RA) (McBurney et al. 1988). Briefly, 1.5 × 106 cells were seeded in a 100 mm diameter bacteriological Petri dish (Fisher Scientific Montreal, QC, Canada) containing 10 mL of differentiation medium [DM: α-MEM supplemented with 5% (v/v) FBS, 5% (v/v) newborn calf serum (Wisent Inc., St-Bruno, QC, Canada), 50 U/mL penicillin, 50 µg/mL of streptomycin, and 0.5 μM all-trans-RA (Sigma-Aldrich, Oakville, ON, Canada)]. After 2 days, the differentiation medium containing RA was refreshed, and the cells were incubated for 2 more days. During the 4 days (D0–D4) of exposure to RA, the cells differentiated while forming floating spheroids in the DM. At D4, the cellular suspensions were collected and incubated at room temperature for about 5 min to allow the sedimentation of the spheroids which were then resuspended in phosphate buffer saline [PBS: NaCl 0.8% (w/v), KCl 0.02% (w/v), KH2PO4 0.02% (w/v), Na2HPO4 0.12% (w/v), and pH 7.3)] and sedimented again. After repeating this procedure for three times, the sedimented spheroids were dissociated by adding 2 mL of 0.025% (v/v) trypsin–1 mM EDTA. Then, 2 mL DM not containing RA was added to stop the reaction. Cell suspension was centrifuged for 1 min at 1000 g and then resuspended in synthetic medium [α-MEM supplemented with 5 µg/mL transferrin, 1 µg/mL insulin (Fisher Scientific Montreal, QC, Canada), 50 U/mL penicillin, and 50 µ/mL streptomycin].
Cell treatment with antihistamine drugs, vDAO and CAT and H2O2
D4 resuspended cells were seeded (3.8 × 105 cells/well) in gelatinized 12-well plates containing 2 mL of synthetic medium and incubated at 37° C in a 5% CO2 and 95% humidified air atmosphere for 2 days (D6). After this period, the culture medium was then renewed, and histamine, DES, CIM, CPX, vDAO, bovine liver catalase (CAT), putrescine (PUT), semicarbazide (SC), or hydrogen peroxide (H2O2) prepared in PBS, were added alone or in association with HA for the times and the final concentrations indicated in the figures. As controls, vDAO and CAT inactivated by heating at 60° C for 10 min (Andrews and Martin 1979; Mondovi et al. 1992) were also used. All agents, except vDAO, were purchased from Sigma-Aldrich (Oakville, ON, Canada). Cell phenotypic analysis, cell viability, and RT-PCR assay of H-receptors were then performed as indicated below.
Cell phenotypic analysis by immunoblotting
For analysis of phenotypic markers, cells differentiated by RA for 4 days (D4) and collected as above reported were seeded in 4 mL of synthetic medium in 60 mm-diameter tissue-grade Petri at densities varying from 1.47 × 106 to 0.37 × 106 cells/Petri according to the duration of the subsequent cultures (D6–D12). Densities were adjusted to obtain similar final cell confluences between cultures while avoiding reseeding which could have disturbed cell culture composition. The synthetic medium was refreshed every 2 days. At different intervals of time, cells were washed in PBS and lysed for 30 min, 4° C, in radioimmunoprecipitation buffer [150 mM NaCl, 50 mM Tris, pH 7.6, 1% (w/v) Nonidet P-40, 0.5% (w/v) deoxycholate, and 0,1% (w/v) sodium dodecylsulfate (SDS) (w/v)] containing 1 mM sodium orthovanadate, 1 mM NaF, and 1/300 protease inhibitors Cocktail-1 (Sigma-Aldrich, Oakville, ON, Canada). The resulting cell protein extracts were analyzed for their protein contents using a bicinchoninic acid microassay kit (Pierce Chemical Co., Rockford, IL, USA), and submitted to SDS-polyacrylamide gel electrophoresis, electron transfer, and immunoblotting procedures as described by Ducharme et al. (2010) with a few modifications. Briefly, protein samples migrated for 15 min at 100 V and 1.25 h at 150 V through 10% polyacrylamide gels and were then transferred from gels onto polyvinylidene difluoride membranes for 35 min at 15 V in transfer buffer [10 mM Tris, 96 mM glycine, 0.01% SDS (w/v), and 20% (v/v) methanol]. After transfer, membranes were washed and incubated with antibodies in TBS-Tw buffer [150 mM NaCl, 50 mM Tris–HCl, pH 7.6, and 0.1% Tween-20 (v/v)] supplemented with 3% (w/v) bovine serum albumin. Primary antibodies were used in the indicated dilutions: mouse IgG1 anti-neurofilament-M (NF-M; 1/1000; Cat. No. 2838; Cell Signaling Technology, Danvers, MA, USA); mouse IgG anti-glial fibrillary acidic protein (GFAP; 1/400; Cat. No. G3893; Sigma-Aldrich, Oakville, ON, Canada); rabbit IgG anti-synaptophysin (SYP; 1/200; Cat. No. sc9116; Santa Cruz Biotechnology, Santa Cruz, CA, USA), and mouse IgG anti-β-Actin (1/10000; Cat. No. A5441; Sigma-Aldrich, Oakville, ON, Canada). Secondary antibodies, conjugated to horseradish peroxidase were from Jackson ImmunoResearch Laboratories, Mississauga, ON, Canada (Cat. Nos. 115–035-062 and 711–035-152; 1/2500 each). Immune complexes were revealed with Immobilon Western Chemiluminescent Substrate (Millipore, Nepean, ON, Canada) and exposed to HyBlot CL films (Denville Scientific Inc., Toronto, ON, Canada) or to a Fusion FX7 imaging system (MBI, Montreal, QC, Canada).
Cell viability assay
The viability of the cells following treatments was measured by the neutral red (NR) assay based on the accumulation of the dye via active transport in the lysosomes of live cells (Perez et al. 2017). At the end of the treatments, above indicated, cells were delicately pre-washed with PBS, and then, 1 mL of 20 mM HEPES, pH 7.4 containing 138 µM NR, 140 mM NaCl, 4 mM KCl, 1.8 mM CaCl2, 0.8 mM MgCl2, and 20 mM D-glucose was added to each culture well. After 2 h of incubation at 37° C and under 5% CO2, the solution was removed, and cells were washed rapidly with 1 mL of solution containing 1% formaldehyde (v/v) and l% CaCl2 (w/v). NR contained in the cells was then extracted by incubation for 10 min, under agitation, with 1 mL of ethanol:H2O:acetic acid solution (50:49:1), and the absorbance read at 540 nm using a microplate reader (Molecular SpectraMax EM Microplate Reader, CA, USA). The viability of cells was calculated as percentages, referring to the absorbance of untreated living cells. All reagents were purchased form Sigma-Aldrich (Oakville, ON, Canada). The half maximal effective concentration (EC50) was estimated as the concentration of HA which induced a response halfway between maximum of viability and the baseline when viability remained constant after a 48 h of exposure to HA.
RT-PCR assay of H-receptors
At the end of exposure time to histamine alone or in association with DES, CIM, or CPX, the total RNA was isolated from the cell pellets using 1 mL TRizol reagent per well (LiTechnologiesies, Gaithersburg, MD, USA). Next, 1–2 μg of RNA was reverse-transcribed to cDNA using the high-capacity cDNA reverse transcription kit (Applied Biosystems, Foster City, CA, USA) and following the manufacturer’s instructions. Real-time polymerase chain reaction (RT-PCR) was then performed to determine the gene expression level of the H1, H2, and H3 receptors using the SsoFast EvaGreen Supermix (Bio-Rad, Hercules, CA, USA), and 30 cycles of amplification in a CFX Connect Real-Time System (Bio-Rad, version 2.1). The resulting amplification products were detected by measuring the fluorescence elicited by the binding of the SYBR Green dye to the double-stranded DNA. QuantiTect primers for H1 (Hrh1_PPM04806B), H2 (Hrh2_PPM04805A), H3 (Hrh3_QT00158375), and H4 (Hrh4_PPM04894A) receptors were purchased from Qiagen (Montreal, QC, Canada). The RPS18 (RRN18S_QT00199367) and peptidylprolyl isomerase A (PPIA_QT01866137) were included as house-keeping genes. Finally, RT-PCR amplicons were electrophoresed, adding molecular weight standards (FroggaBio, Concord, ON, Canada) on a 2% agarose gel, visualized with GreenGlow™ (Denville Scientific Inc., Saint-Laurent, QC, Canada) and using a ChemiDoc MP Imaging System (Bio-Rad, Hercules, CA, USA).
Cellular Ca2+ mobilization
Cells, grown in synthetic medium for 2 days (D6), were exposed for 1 h to Hank’s Balanced Salt Solution containing 10 mM HEPES, pH 7.2 (HBBS-HEPES) and 1 µM Fluo-4 acetoxymethyl ester calcium probe (Fluo-4 AM; Thermo Fisher Scientific, Montreal, QC, Canada), 1 µM pluronic acid F127 (Thermo Fisher Scientific, Montreal, QC, Canada), and 2.5 mM probenecid (Sigma-Aldrich, Oakville, ON, Canada-Aldrich) at 37° C in a 5% CO2-95% humidified air atmosphere. Then, the cells were washed three times with HBSS-HEPES and maintained in 1 mL of the same buffer for 30 min. Cells were then added with 100 µM histamine in the absence or presence of 5 µM DES, 0.1 µM CIM, or 0.4 µM vDAO, and fluorescent images were acquired by a confocal microscopy by measuring the fluorescence of Fluo4-Ca2+ signal at 525 nm, upon excitation at 488 nm. Nikon A1 confocal microscope equipped with a 10X objective (Nikon Canada, Mississauga, ON, Canada) was used. The acquisition was operated in kinetic scan mode, at 2.5 min with a rate of 2 s per scan, a pinhole diameter of 11.9 µm, a detector sensibility or high voltage of 45, and an offset of -5. The ImageJ software (Wayne Rasband National Institutes of Health, USA) was used to quantify the Ca2+-fluorescence. Results are expressed as area under the curve (AUC) for the first 120 s following the addition of HA.
H2O2 spectrofluorimetric assay
The generation of H2O2 was measured using a spectrofluorimetric assay based on the oxidation of homovanillic acid (HVA) under the catalysis of horseradish peroxidase (HRP) forming a fluorescent dimer in the presence of H2O2 (Baggiolini et al. 1986). Briefly, Day 6 P19 neuron cultures, obtained as described above, were delicately pre-washed with PBS and received 1.75 mL of a reaction mixture containing HVA (100 µM)-HRP (1U/mL) in HBSS. The reaction was started by the addition of 0.25 mL of HA (100 µM in HBSS) alone or in association with DES (5 µM), CIM (1 µM) or CPX (0.01 µM). The blank control cultures received HBSS alone and the positive control cultures received HBSS containing 10 nmol H2O2. Cultures treated with DES (5 µM), CIM (1 µM), or CPX (0.01 µM) in the absence of HA were also included for comparison. After a 4 h of incubation at 37 °C, 5% CO2, the reaction was stopped with 0.25 mL of 0.1 M NaOH (pH 12). The reaction mixtures were collected and centrifuged for 10 min at 1200 g, and their fluorescence measured at 25 °C in a PerkinElmer LS45 fluorimeter (PerkinElmer, Waltham, MA, USA), using 315 nm (excitation) and 425 nm (emission) wavelengths. The standard curves were performed in the absence of cells. The standard solutions were prepared by the addition of 0.25 mL HBSS alone (blank) or containing 0.1 to 10 nmol H2O2, to 1.75 mL of HBSS containing HVA (100 µM)-HRP (1U/mL). The reaction was stopped after 4 h by the addition of 0.25 mL of 0.1 M NaOH. The fluorescence value of each culture sample and standard solution was corrected from that of its own blank before its report to the standard curve. Upon verification, there was no background fluorescence in these additional blanks: H2O2 ± cells, HVA ± cells, HRP ± cells, (HVA + HRP) ± cells, (H2O2 + HVA) ± cells, and (H2O2 + HRP) ± cells.
Statistics
All experiments used a minimum of three replicates. Where relevant, data are expressed as the mean ± SD. Statistical tests were performed with the GraphPad software, using either one-way, two-way ANOVA (for comparison between three groups) or two-tailed Student’s t test (for comparison between two groups). Differences were deemed statistically significant when the associated P value was lower than 0.05 (P < 0.05).
Results and discussion
Differentiation of cultured P19 cells into neurons by retinoic acid
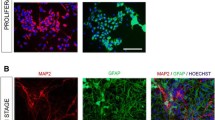
The treatment of mouse P19 embryonal carcinoma cells with RA for 4 days have been reported to induce their differentiation into a population of cells consisting of neurons and other cell types, such as fibroblasts and glial cells. Neurons express a variety of neuronal markers, develop neurite processes presenting characteristics of axons and dendrites, and form synapses (Staines et al. 1994; Poirier et al. 2006). Immunoblotting analysis of NF-M neuronal marker showed the presence of neurons from D6 to D12, the last day analyzed (Fig. 1).
Neurons mature in these cultures as indicated by the increasing levels of SYP synaptic marker (Fig. 1). The GFAP glial marker was almost undetectable until D10 indicating a very low number, if not the absence of astrocytes in the cell populations during this time interval (Fig. 1) This is in line with our reported immunofluorescence studies, showing that D10 RA-treated cultures contain mainly neurons, since about 95% of the cells expressed the neuronal nuclear marker NeuN and about 5% were GFAP positive (Poirier et al. 2006). As our objective was to characterize the response to histamine of cultured P19 neurons in absence of astrocytes, the following studies were carried out with D6 cultures. This is pertinent to the fact that astrocytes participate in the regulation of histamine metabolism and function (Jurič et al. 2016; Otsuka and Naganuma 2022).
Loss of cell viability in P19 neurons treated with histamine
The incubation of D6 P19 neurons with histamine for 48 h caused a loss of viability (Fig. 2A). Indeed, viability dropped to 65% in a histamine-dependent concentration for up to 100 µM histamine. The EC50 value of this histamine effect was 54.1 ± 1.2 µM, that is the concentration for the median value between 100 and 65% viability (or between 0 and 35% mortality). This calculation considers that the loss of viability remained constant (64 ± 2%) for concentrations equal to or higher than 100 µM histamine. This behavior differs from what was previously observed with Caco-2 cells, in which the loss of viability consistently increased with the concentration (Jumarie et al. 2017).
Effect of histamine on P19 neurons viability: A D6 P19 neurons were treated for 48 h with various concentrations of histamine (HA) and analyzed for viability. The dotted lines show the linear relationship of histamine action at concentrations ≤ 100 µM, and the plateau effect at higher concentrations. B Time-course of P19 neurons viability upon exposure to 100 µM histamine at D6. Data are means ± SD of three independent experiments and reported as percentages of untreated control cultures
The results here reported suggest that 65% of the cells remained unaffected or cannot respond to histamine action. This would be in line with the fact that, like primary neuronal cultures, P19 neuronal cultures contain mixed populations of neurons (McBurney et al. 1988). Another possibility is that the H-receptors are progressively impacted by the amount of histamine administered. When the whole amount of H-receptor is saturated, a larger amount of histamine would not generate a further decrease of viability. Accordingly, in the present work, 100 µM histamine was chosen as the reference concentration to carry out subsequent tests.
The time-dependent effect of 100 µM histamine was also analyzed. As reported in Fig. 2B, histamine caused a marked linear decrease in cell viability during the first 4 h of exposure. Then, cell viability remained constant (about 65%) for up to 48 h. It is not excluded that the first step of HA impacting the H-receptors was followed by a period related to an arrest of signalization. The effect of histamine on neuronal viability could involve its metabolites or its receptors. This possibility has been next evaluated.
No involvement of DAO activity in the histamine-induced loss of cell viability
The histamine effect on the viability of P19 neurons could have been related to: (i) the oxidative deamination of this biogenic amine by an endogenous DAO which would have produced cytotoxic H2O2, imidazole-4-acetaldehyde and NH3 or (ii) the activation of H-receptors leading to the release of ROS, and nitric oxide (NO). To investigate the possible involvement of an endogenous DAO, P19 neurons were treated with putrescine (PUT) and semicarbazide (SC) (Fig. 3). The DAO can metabolize not only histamine but also putrescine (the substrate for which DAO displays the highest rate of enzymatic oxidation (Pietrangeli et al. 2007)). Also, as a copper enzyme, DAO is inhibited by semicarbazide (Obata. 2002).
Effect of putrescine and semicarbazide in relation with the action of histamine on P19 neurons viability. Cells were incubated for 4 h in the presence of histamine (HA) or putrescine (PUT) at different concentrations (A), or in the presence of 100 µM of histamine with or without semicarbazide (SC) at various concentrations (B). Data are means ± SD of three independent experiments in duplicate and reported as percentages of untreated control cultures (n = 3; ns non-significant; *P < 0.05, **P < 0.01, ***P < 0.001; two-way ANOVA multiple comparisons)
Figure 3A presents the comparative effects on cell viability of HA and PUT used at the same concentrations. PUT did not affect the viability of cells despite its higher degradation by DAO. As already observed above, the viability of cells decreased to 65% for histamine concentrations ≥ 0.1 mM. On the contrary, PUT presented good biocompatibility, preserving cell viability even at concentrations up to 5 mM. These data suggest that the decreased viability was specifically related to histamine only and not to putrescine. Moreover, if an endogenous copper-containing DAO was present on P19 neurons, this DAO would have modulated the viability of P19 neurons, and this modulation would have been modified in the presence of SC known as a specific inhibitor of copper-containing enzymes and is largely used to confirm the presence of these enzymes in biological systems (Obata. 2002).
Practically, Fig. 3B shows that SC itself (1–10 mM) was not toxic to cells. Furthermore, SC did not prevent the loss of cell viability induced by histamine since the viability remained at about 65%. Therefore, the results with SC provided an argument supporting the absence of an endogenous DAO and agreed with other data showing that DAO expression in the CNS is low or absent (Yoshikawa et al. 2019). Data of this section indicated that the toxic effect of histamine was not related to its degradation by an endogenous DAO but involved other cellular mechanisms such as activation of receptors.
Involvement of H-receptors in histamine-induced loss of cell viability
To evaluate the involvement of H-receptors in histamine-induced loss of cell viability, P19 neurons were treated with H1 (DES), H2 (CIM), or H3 (CPX) antagonists for 4 h, in the presence of 100 µM histamine (Fig. 4). DES is known as a non-competitive antihistamine agent acting on H1 by binding to a site different from that of histamine-binding site (Agrawal 2001) and CIM is a competitive inhibitor of H2 (Jafarzadeh et al. 2019). CPX is considered as a potent and selective H3 antagonist/inverse agonist that binds to the histamine-binding site on the receptor (Leurs et al. 2005). None of the three antihistamine agents exerted a toxic effect by themselves (Fig. 4).
Effect of histamine (HA, 100 µM) on cell viability after 4 h of treatment in the absence or presence of the antihistamine agents: Desloratadine (DES) (A), Cimetidine (CIM) (B), and Ciproxifan (CPX) (C). Notice the absence of an effect on viability for DES, CIM, and CPX used alone (A, B, C). Data are means ± SD of three independent experiments in duplicate (n = 3; *P < 0.05, **P < 0.01, ***P < 0.001; two-way ANOVA multiple comparisons)
Both DES and CIM decreased the histamine-induced loss of viability in an apparent concentration-dependent manner, with a complete abolition of the cytotoxic effect at concentrations greater than 0.1 µM (Fig. 4A, B). The apparent EC50 values for this protection were about 0.044 ± 0.01 µM and 0.059 ± 0.005 µM, respectively. Differently, CPX did not alleviate the loss of cell viability triggered by histamine (Fig. 4C). These results point to the activation of H1 and H2, but not H3, as being involved in the neuronal cytotoxic effect of histamine.
Histamine-induced cellular Ca2+ mobilization
The activation of H1 and H2 has previously been reported to trigger Ca2+ mobilization from the endoplasmic reticulum stores (Jones and Kearns 2011) through the activation of the phospholipase-C pathway (Panula et al. 2015; Obara et al. 2020). The activation of Gαi/o proteins may lead to the mobilization of intracellular Ca2+ levels upon the activation of H3 (Bongers et al. 2007). Since, the H3 receptor did not seem to be involved in the loss of neuron viability by histamine stimulus (Fig. 4C), the modulation of Ca2+ mobilization under histamine stimulation by CPX was not included. The concentrations used of DES and CIM were higher than their respective Kd values (Kd = 1.1 for DES (Anthes et al. 2002); Kd = 0.8 μM for CIM (Khateb et al. 1995), to afford a possible saturation of receptors by these antihistamine agents. The fluorescence measurements of intracellular Ca2+ level showed that Ca2+ mobilization was induced by exposure to histamine (Fig. 5A). This was significantly attenuated when cells were co-treated with histamine and the antihistamine DES (Fig. 5B, C) or CIM (Fig. 5B, D). These results support the hypothesis of the presence of H1 and H2 in P19 neurons and their involvement in histamine-induced Ca2+ mobilization. The Ca2+ signal induced by histamine was also lowered by the addition of vDAO (Fig. 5B, E) which should be due to the oxidative decomposition of histamine by this enzyme. Overall, it appears that P19 neurons express functional H1 and H2 receptors and their inhibition or blockage is sufficient to abolish the histamine-induced loss of neuronal viability.
Effect of histamine, antihistamine drugs, and vDAO on intracellular calcium levels in P19 neurons. Neurons were pre-loaded with the Fluo-4AM calcium probe before treatment with the different agents. Time-course of fluorescence profile related to Ca2+ mobilization induced by histamine (HA, 100 µM) stimulation alone (A) or following a 180 s pretreatment with DES (5 µM), CIM (1 µM), or vDAO (0.4 µM) (B) as measured by real-time confocal microscopy. The quantified area under the curve (AUC) was obtained by ImageJ software (C, D, E). Data are expressed as means ± SD of at least three experimental runs in duplicate (n = 3; ***P < 0.001; two-way ANOVA multiple comparisons)
Expression of H1, H2, and H3 receptors in P19 neurons
We used RT-PCR to address whether P19 neurons do express H-receptors (Fig. 6). The presence of H1, H2, and H3 was clearly revealed by the generation of specific amplicons of the expected sizes (73 bp for H1, 137 bp for H2, and 87 bp for H3). The expression of the H1 receptor has been reported in undifferentiated P19 cells (Bloemers et al. 1993), but, to the best of our knowledge, this is the first report of the expression of H1, H2, and H3 receptors in P19 neurons. On the contrary, H4 seemed to not be expressed in view of the absence of specific amplicons (84 bp for H4) (Fig. 6D).
Agarose gels of H1 (A), H2 (B), H3 (C), and H4 (D) amplicons obtained by RT-PCR analysis. P19 neurons were incubated for 4 h with histamine (HA, 100 µM) in the absence or presence of DES (1 µM), CIM (0.1 µM), or CPX (0.01 µM). The 18S was used as endogenous RNA standard and peptidylprolyl isomerase A (PPIA) as an internal standard (E). The gels are representative of three repeated experiments
Modulation of histamine effect on P19 neurons by exogenous diamine oxidase and catalase
To evaluate whether the effect of histamine on cell viability is derived from the H2O2 produced from its own interaction with H-receptors, we next treated cultured P19 neurons with vDAO (acting on histamine) or with CAT (decomposing H2O2). Figure 7A shows that vDAO alone, whether active or inactivated, did not alter cell viability. When associated to histamine, vDAO under its active form (even at nanomolar concentrations), almost completely canceled the toxic effect of histamine. This protection tended to slightly diminish at higher concentrations, possibly due to the production of H2O2 by its oxidase reaction, and was abolished in the case of inactivated vDAO, indicating that the involvement of its enzymatic activity is requested for this protection.
Effect of vDAO and CAT on histamine-induced loss of neuron viability. P19 neurons were treated for 4 h with 100 µM histamine (HA) in the absence or presence of active or heat-inactivated vDAO (A) or CAT (B), at various concentrations. The results (means ± SD of three independent experiments in duplicate) are reported as percentages of untreated cells (n = 3; **P < 0.01, ***P < 0.001; two-way ANOVA multiple comparisons
Figure 7B shows that active and inactivated CAT alone did not affect cell viability and that active CAT protected P19 neurons against histamine toxicity. These results suggest an involvement of H2O2 generated by the cells in histamine toxicity. The activation of H-receptors triggers a series of reactions leading to the generation of ROS, NO, and H2O2 that are detrimental to cells, since they can cause in situ oxidative damage to cell structures or components, such as membranes, proteins, and DNA. Previous studies have shown that histamine induced the production of ROS in N9 microglial cell line and in primary murine microglia cells via the activation of H-receptors by 100 μM histamine (Rocha et al. 2016). This concentration was the same as that used in this report. Also, Medina et al. (2009) showed that histamine (100 μM) stimulated the production of H2O2 in WM35 melanoma cells and this effect was reversed by CAT treatment.
To investigate if histamine should stimulate the production of H2O2 in P19 neurons, these cells were treated with histamine (100 µM) alone or in association with antihistamine drugs, vDAO or CAT (Fig. 8). The fluorometric measure of oxidation product of HVA catalyzed by HRP was used to quantify the H2O2 produced by cells. The medium recuperated from cells treated with histamine (100 µM) contained 8.36 ± 0.08 nmol of H2O2 (Fig. 8A, B). An important reduction of the amount of H2O2 was observed when cells were exposed to histamine in association with DES or CIM at the concentrations showed in Fig. 8A. In contrast, CPX (H3 antagonist) was not able to reduce the generation of H2O2 (Fig. 8A). The inhibition or blockage of H1 and H2 receptors by DES or CIM, respectively, was also sufficient to avoid the loss of neuronal viability (Fig. 4A, B) as well as the reduction of Ca2+ signal, both induced by histamine (Fig. 5A–D).
Production and toxic effect of the H2O2 in P19 neuronal cultures. Determination of H2O2 released in vitro by P19 neurons after 4 h of treatment with histamine (HA, 100 µM) in the presence or in the absence of the antihistamine agents: A Desloratadine (DES), Cimetidine (CIM), or Ciproxifan (CPX), or B Catalase (CAT) or vDAO, using a H2O2 standard curve (inset in A). The 10 nmol H2O2 standard were included as positive control. It was noticed the low levels of H2O2 production when DES, CIM, CPX, CAT, and vDAO were used alone. C Effect of H2O2 (0–15 nmol) on cell viability. The results are means ± SD of three independent experiments done in duplicate (n = 3; ***P < 0.001; one-way ANOVA multiple comparisons)
The vDAO and CAT also decreased the production of H2O2 (Fig. 8B). CAT could protect cells against histamine-mediated loss of viability by scavenging H2O2, while exogenous vDAO, although a generator of H2O2, could provide protection by decreasing the amount of histamine capable of binding to H-receptors. In aerobic organisms, CAT, as an enzyme involved in the scavenging of ROS, transforms H2O2 into molecular oxygen and H2O. It is important to note that H2O2 is a stable nonradical oxidative species that can easily cross biological membranes and move from the interior to the exterior of the cell (Flora 2009) where it can be decomposed by CAT (Medina et al. 2009). The viability of P19 neurons is affected by H2O2 quantities as those produced by histamine treatment (Fig. 8C).
Conclusions
The results provide evidence that P19 neurons express functional H1, H2, and H3 and that their exposure to histamine results in a loss of cell viability. The observed histamine-dependent cytotoxic effect was prevented by the treatment with DES and CIM (H1 and H2 antagonists) as well as by the addition of vDAO or CAT but not by H3 antagonist. The H4 seemed to not be expressed on these cells. The vDAO likely protects by decreasing extracellular histamine levels, and CAT by scavenging H2O2 escaped from the cells following activation of H-receptors. Also, DES and CIM can significantly reduce the amount of H2O2 released by cells treated with histamine. P19 neurons appear as sensitive to histamine (being affected by low concentrations of this compound), pointing to their usefulness as a pertinent model in the search for histamine roles in neuronal system. Furthermore, P19 neurons, easy to cultivate, can be used in the investigation of pharmaceuticals such as antihistamine agents, and neuropharmaceuticals, particularly for histamine-related neuronal dysfunctions. As a perspective, they can be considered in the biopharmaceuticals studies to evaluate novel antihistamine drugs or the association of antihistamine drugs with other bioactive agents in histamine neural neuronal pathogenesis. The major advantages of the proposed approach consist in the fact that various antihistamine drugs can be simply and directly evaluated in the presence of histamine. The robustness of P19 neurons together with their easy handling make the P19 neuronal model an interesting choice for rapid in vitro screening of antihistamine drugs. This model presents properties like those of cultured mammalian brain and it is less expensive to operate than primary cell cultures. This model can be easily implemented by current laboratories of analysis and by Contract Research Organizations (CROs).
Abbreviations
- AUC:
-
Area under the curve
- Ca2+ :
-
Calcium ions
- CAT:
-
Catalase
- CIM:
-
Cimetidine
- CNS:
-
Central nervous system
- CPX:
-
Ciproxifan
- D:
-
Day
- DAO:
-
Diamine oxidase
- vDAO:
-
Vegetal DAO
- DES:
-
Desloratadine
- DM:
-
Differentiation medium
- ENS:
-
Enteric nervous system
- FBS:
-
Fetal bovine serum
- GFAP:
-
Glial fibrillary acidic protein
- H1 :
-
Histamine H1 receptor
- H2 :
-
Histamine H2 receptor
- H3 :
-
Histamine H3 receptor
- H4 :
-
Histamine H4 receptor
- H-receptor:
-
Histamine receptors
- HA:
-
Histamine
- HBSS:
-
Hank’s balanced salt solution
- H2O2 :
-
Hydrogen peroxide
- HEPES:
-
4-(2-Hydroxyethyl)-1-piperazineethanesulfonic acid
- HRP:
-
Horseradish peroxidase
- HVA:
-
Homovanilic acid
- NF-M:
-
Neurofilament M
- NO:
-
Nitric oxide
- NR:
-
Neutral red
- PBS:
-
Phosphate-buffered saline
- PUT:
-
Putrescine
- RA:
-
Retinoic acid
- ROS:
-
Reactive oxygen species
- RT-PCR:
-
Real-time polymerase chain reaction
- SC:
-
Semicarbazide
- SDS:
-
Sodium dodecylsulfate
- SYP:
-
Synaptophysin
References
Agrawal DK (2001) Pharmacology and clinical efficacy of desloratadine as an anti-allergic and anti-inflammatory drug. Expert Opin Investing Drugs 10:547–560. https://doi.org/10.1517/13543784.10.3.547
Andrews GP, Martin SE (1979) Heat inactivation of catalase from Staphylococcus aureus MF-31. Appl Environ Microbiol 37:1180–1185. https://doi.org/10.1128/aem.37.6.1180-1185.1979
Anthes JC, Gilchrest H et al (2002) Biochemical characterization of desloratadine, a potent antagonist of the human histamine H(1) receptor. Eur J Pharmacol 449:229–237. https://doi.org/10.1016/S0014-2999(02)02049-6
Baggiolini M, Walter R, Philip HC (1986) [22] Measurement of hydrogen peroxide production by phagocytes using homovanillic acid and horseradish peroxidase. Methods Enzymol 132:395–400. https://doi.org/10.1016/S0076-6879(86)32024-X
Blemur L, Le TC, Marcocci L, Pietrangeli P, Mateescu MA (2016) Carboxymethyl starch/alginate microspheres containing diamine oxidase for intestinal targeting. Biotechnol Appl Biochem 63:344–353. https://doi.org/10.1002/bab.1369
Bloemers SM, Leurs R, Smit MJ, Verheule S, Tertoolen LGJ, Timmerman H, Delaat SW (1993) Mouse P19 embryonal carcinoma cells express functional histamine H1 receptors. Biochem Biophys Res Comm 191:118–125. https://doi.org/10.1006/bbrc.1993.1192
Bongers G, Bakker RA, Leurs R (2007) Molecular aspects of the histamine H3 receptor. Biochem Pharmacol 73:1195–1204. https://doi.org/10.1016/j.bcp.2007.01.008
Branco ACCC, Yoshikawa FSY, Pietrobon AJ, Sato MN (2018) Role of histamine in modulating the immune response and inflammation. Mediators Inflamm doi: https://doi.org/10.1155/2018/9524075
Breunig E, Michel K, Zeller F, Seidl S, Weyhern CWHV, Schemann M (2007) Histamine excites neurons in the human submucous plexus through activation of H1, H2, H3 and H4 receptors. J Physiol 583:731–742
Brown RE, Stevens DR, Haas HL (2001) The physiology of brain histamine. Prog Neurobiol 63:637–672. https://doi.org/10.1016/S0301-0082(00)00039-3
Canonica GW, Blaiss M (2011) Antihistaminic, anti-inflammatory, and antiallergic properties of the nonsedating second generation antihistamine desloratadine: a review of the evidence. The World Allergy Organ J 4:47–53. https://doi.org/10.1097/WOX.0b013e3182093e19
Duan H, Wang J (2010) Selective transport of monoamine neurotransmitters by human plasma membrane monoamine transporter and organic cation transporter 3. J Pharmacol Exp Ther 33:743–753. https://doi.org/10.1124/jpet.110.170142
Ducharme P, Maltais D, Desroches D, Mateescu MA, Paquin J (2010) Ceruloplasmin induced aggregation of P19 neurons involves a serine protease activity and is accompanied by reelin cleavage. Neuroscience 167:633–643. https://doi.org/10.1016/j.neuroscience.2010.02.039
Flora SJ (2009) Structural, chemical and biological aspects of antioxidants for strategies against metal and metalloid exposure. Oxid Med Cell Longev 2:191–206. https://doi.org/10.4161/oxim.2.4.9112
Furness JB (2000) Types of neurons in the enteric nervous system. J Auton Nervous Syst 81:87–96
Haas HL, Sergeeva OA, Selbach O (2008) Histamine in the nervous system. Physiol Rev 88:1183–1241. https://doi.org/10.1152/physrev.00043.2007
Halliwell B (2007) Biochemistry of oxidative stress. Biochem Soc Trans 35:1147–1150. https://doi.org/10.1042/bst0351147
Huang H, Li Y, Liang JF, Finkelman D (2018) Molecular regulation of histamine synthesis. Front Immunol 9:1392. https://doi.org/10.3389/fimmu.2018.01392
Jafarzadeh A, Nemati M, Khorramdelazad H, Hassan ZM (2019) Immunomodulatory properties of cimetidine: Its therapeutic potentials for treatment of immune-related diseases. Int Immunopharmacol 70:156–166. https://doi.org/10.1016/j.intimp.2019.02.026
Jones B, Kearns G (2011) Histamine: new thoughts about a familiar mediator. Clin Pharm Therap 89:189–197. https://doi.org/10.1038/clpt.2010.256
Jumarie C, Séïde M, Marcocci L, Pietrangeli P, Mateescu MA (2017) Diamine oxidase from white pea (Lathyrus sativus) combined with catalase protects the human intestinal Caco-2 cell line from histamine damage. Appl Biochem Biotechnol 182:1171–1181. https://doi.org/10.1007/s12010-016-2390-3
Jurič DM, Kržan M, Lipnik-Stangelj M (2016) Histamine and astrocyte function. Pharmacol Res 111:774–783
Kemp SF, Lockey RF (2002) Anaphylaxis: a review of causes and mechanisms. J Allergy Clin Immunol 110:341–348. https://doi.org/10.1067/mai.2002.126811
Khateb A, Fort P, Pegna A, Jones BE, Mühlethaler M (1995) Cholinergic nucleus basalis neurons are excited by histamine in vitro. Neuroscience 69:495–506. https://doi.org/10.1016/0306-4522(95)00264-J
Kulkarni S, Ganz J, Bayrer J, Becker L, Bogunovic M, Rao M (2018) Advances in enteric neurobiology: The “brain” in the gut in health and disease. J Neurosci 38:9346–9354. https://doi.org/10.1523/JNEUROSCI.1663-18.2018
Leurs R, Bakker RA, Timmerman H, de Esch IJ (2005) The histamine H3 receptor: from gene cloning to H3 receptor drugs. Nat Rev Drug Discov 4:107–120. https://doi.org/10.1038/nrd1631
Leurs R, Hough L, Blandina P, Haas HL (2012) Histamine in: Basic Neurochemistry, Academic Press. pp. 323–341. https://doi.org/10.1016/B978-0-12-374947-5.00016-X
Maintz L, Novak N (2007) Histamine and histamine intolerance. Am J Clin Nutr 85:1185–1196. https://doi.org/10.1093/ajcn/85.5.1185
Masini E, Bani D, Marzocca C, Mateescu MA, Mannaioni PF, Federico R, Mondovi B (2007) Pea seedling histaminase as a novel therapeutic approach to anaphylactic and inflammatory disorders. Sci World J 7:888–902
McBurney MW, Reuhl KR, Ally AI, Nasipuri S, Bell JC, Craig J (1988) Differentiation and maturation of embryonal carcinoma-derived neurons in cell culture. J Neurosci 8:1063–1073. https://doi.org/10.1523/JNEUROSCI.08-03-01063.1988
Medina VA, Massari NA, Cricco GP, Martín GA, Bergoc RM, Rivera ES (2009) Involvement of hydrogen peroxide in histamine-induced modulation of WM35 human malignant melanoma cell proliferation. Free Radic Biol Med 46:1510–1515. https://doi.org/10.1016/j.freeradbiomed.2009.03.003
Mittal R, Debs LH et al (2017) Neurotransmitters: The critical modulators regulating gut–brain axis. J Cell Physiol 232:2359–2372. https://doi.org/10.1002/jcp.25518
Molina-Hernández A, Velasco I (2008) Histamine induces neural stem cell proliferation and neuronal differentiation by activation of distinct histamine receptors. J Neurochem 106:706–717. https://doi.org/10.1111/j.1471-4159.2008.05424.x
Mondovi B, Befani O, Gerosa P, Mateescu MA (1992) Specific temperature dependence of diamine oxidase activity and its thermal stability in the presence of polyvinylalcohol. Agents Actions 37:220–226. https://doi.org/10.1007/BF02028112
Mondovi B, Fogel WA, Federico R, Calinescu C, Mateescu MA, Rosa AC, Masini E (2013) Effects of amine oxidases in allergic and histamine mediated conditions. Recent Pat Inflamm Allergy Drug Discov 7:20–34
Neree AT, Soret R, Marcocci L, Pietrangeli P, Pilon N, Mateescu MA (2020) Vegetal diamine oxidase alleviates histamine-induced contraction of colonic muscles. Sci Rep 10:1–13. https://doi.org/10.1038/s41598-020-78134-3
Obara I, Telezhkin V, Alrashdi I, Chazot PL (2020) Histamine, histamine receptors, and neuropathic pain relief. Br J Pharmacol 177:580–599. https://doi.org/10.1111/bph.14696
Obata T (2002) Semicarbazide sensitive amine oxidase (SSAO) in the brain. Neurochem Res 27:263–268
Otsuka R, Naganuma F et al (2022) Contribution of astrocytic histamine N-methyltransferase to histamine clearance and brain function in mice. Neuropharmacology. 212: 109065. https://doi.org/10.1016/j.neuropharm.2022.109065
Panula P (2021) Histamine receptors, agonists, and antagonists in health and disease. Handb Clin Neurol 180:377–387. https://doi.org/10.1016/B978-0-12-820107-7.00023-9
Panula P, Chazot PL, Cowart M, Gutzmer R, Leurs R, Liu WL, Stark H, Thurmond RL, Haas HL (2015) International union of basic and clinical pharmacology. XCVIII Histamine Receptors Pharmacol Rev 67:601–655. https://doi.org/10.1124/pr.114.010249
Perez MG, Fourcade L, Mateescu MA, Paquin J (2017) Neutral Red versus MTT assay of cell viability in the presence of copper compounds. Anal Biochem 535:43–46. https://doi.org/10.1016/j.ab.2017.07.027
Pietrangeli P, Federico R, Mondovì B, Morpurgo L (2007) Substrate specificity of copper containing plant amine oxidases. J Inorg Biochem 101:997–1004. https://doi.org/10.1016/j.jinorgbio.2007.03.014
Pietrangeli P, Capuozzo E, Mateescu MA, Marcocci L (2020) Copper-containing amine oxidase purified from Lathyrus sativus as a modulator of human neutrophil functions. Int J Mol Med 45:1583–1590. https://doi.org/10.3892/ijmm.2020.4535
Poirier S, Prat A, Marcinkiewicz E, Paquin J, Chitramuthu BP, Baranowski D, Cadieux B, Bennett HP, Seidah NG (2006) Implication of the proprotein convertase NARC-1/PCSK9 in the development of the nervous system. J Neurochem 98:838–850. https://doi.org/10.1111/j.1471-4159.2006.03928.x
Rocha SM, Pires J, Esteves M, Graça B, Bernardino L (2014) Histamine: a new immunomodulatory player in the neuron glia crosstalk. Front Cell Neurosci 8:120. https://doi.org/10.3389/fncel.2014.00120
Rocha SM, Saraiva T et al (2016) Histamine induces microglia activation and dopaminergic neuronal toxicity via H1 receptor activation. J Neuroinflamm 13:1–16. https://doi.org/10.1186/s12974-016-0600-0
Schwartz JC, Arrang JM, Garbarg M, Pollard H, Ruat M (1991) Histaminergic transmission in the mammalian brain. Physiol Rev 71:1–51. https://doi.org/10.1152/physrev.1991.71.1.1
Shahid M, Tripathi T, Sobia F, Moin S, Siddiqui M, Khan RA (2009) Histamine, histamine receptors, and their role in immunomodulation: an updated systematic review. Open Immunol J 2:9–41. https://doi.org/10.2174/1874226200902010009
Spencer NJ, Hu H (2020) Enteric nervous system: sensory transduction, neural circuits and gastrointestinal motility. Nat Rev Gastroenterol Hepatol 17:338–351. https://doi.org/10.1038/s41575-020-0271-2
Staines WA, Morassutti DJ, Reuhl KR, McBurney MW (1994) Neurons derived from P19 embryonal carcinoma cells have varied morphologies and neurotransmitters. Neuroscience 58:735–775. https://doi.org/10.1016/0306-4522(94)90451-0
Tiligada E, Kyriakidis K, Chazot PL, Passani MB (2011) Histamine pharmacology and new CNS drug targets. CNS Neurosci Ther 17:620–628. https://doi.org/10.1111/j.1755-5949.2010.00212.x
Ulrich H, Majumder P (2006) Neurotransmitter receptor expression and activity during neuronal differentiation of embryonal carcinoma and stem cells: from basic research towards clinical applications. Cell Prolif 39:281–300. https://doi.org/10.1111/j.1365-2184.2006.00385.x
Yoshikawa T, Nakamura T, Yanai K (2019) Histamine N-methyltransferase in the brain. Int J Mol Sci 20:737. https://doi.org/10.3390/ijms20030737
Yuan H, Silberstein SD (2018) Histamine and migraine. Headache 58:184–193. https://doi.org/10.1111/head.13164
Acknowledgements
This project was supported by the “Fondation Courtois” (Québec, Canada) and by grants from the Natural Sciences and Engineering Research Council (NSERC) of Canada to MAM and to BA. MAM holds an Institutional Research Chair in Enteric Dysfunctions and BA holds an Institutional Research Chair in Cancer Prevention and Treatment. MGP was granted a studentship from CERMO-FC, GT received an NSERC Undergraduate Student Research Award, ATN was a holder of a doctoral scholarship from the Fonds de la Recherche du Québec Santé (FRQS, Québec, Canada) and of a Mobility Fellowship from CRIPA/FRQNT and NGS was holder of a doctoral scholarship from FRQNT.
Funding
The work of MAM, JP and BA was supported by the Natural Sciences and Engineering Research Council (NSERC) of Canada under Grant No. 06919, 105730 and 06651, respectively.
Author information
Authors and Affiliations
Contributions
Conceptualization, MGP, MAM, and JP; methodology, MGP, GT, ATN, NGS, CL, JP, LM, PP, BA, and MAM; investigation, MGP, GT, ATN, NGS, CL, JP, BA, and MAM; writing—original draft preparation, MGP, JP, LM, PP, BA, and MAM; writing—review and editing, MGP, JP, LM, PP, BA, and MAM.; supervision, MAM; project administration, MAM; funding acquisition, MAM, JP and BA. All authors have read and agreed to the published version of the manuscript.
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that there is no conflict of interest.
Additional information
Handling editor: V. Bolshakov.
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Perez, M.G., Tanasie, G., Neree, A.T. et al. P19-derived neuronal cells express H1, H2, and H3 histamine receptors: a biopharmaceutical approach to evaluate antihistamine agents. Amino Acids 55, 821–833 (2023). https://doi.org/10.1007/s00726-023-03273-6
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00726-023-03273-6