Abstract
Carnosine and other histidine-containing dipeptides are expected to be important anti-oxidants in vertebrates based on various in vitro and in vivo studies with exogenously administered carnosine or its precursor β-alanine. To examine a possible anti-oxidant role of endogenous carnosine, mice lacking carnosine synthase (Carns1−/−) had been generated and were examined further in the present study. Protein carbonylation increased significantly between old (18 months) and aged (24 months) mice in brain and kidney but this was independent of the Carns1 genotype. Lipoxidation end products were not increased in 18-month-old Carns1−/− mice compared to controls. We also found no evidence for compensatory increase of anti-oxidant enzymes in Carns1−/− mice. To explore the effect of carnosine deficiency in a mouse model known to suffer from increased oxidative stress, Carns1 also was deleted in the type II diabetes model Leprdb/db mouse. In line with previous studies, malondialdehyde adducts were elevated in Leprdb/db mouse kidney, but there was no further increase by additional deficiency in Carns1. Furthermore, Leprdb/db mice lacking Carns1 were indistinguishable from conventional Leprdb/db mice with respect to fasting blood glucose and insulin levels. Taken together, Carns1 deficiency appears not to reinforce oxidative stress in old mice and there was no evidence for a compensatory upregulation of anti-oxidant enzymes. We conclude that the significance of the anti-oxidant activity of endogenously synthesized HCDs is limited in mice, suggesting that other functions of HCDs play a more important role.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
The dipeptide carnosine (β-alanyl-l-histidine) and related histidine-containing dipeptides (HCDs) have been found in all mammals analysed and in many vertebrates (Boldyrev et al. 2013). Carnosine is synthesized by an amino acid ligase, carnosine synthase, encoded by the carnosine synthase 1 gene (Carns1; EC 6.3.2.11), also known as ATP-grasp domain containing 1 gene (Drozak et al. 2010; Kwiatkowski et al. 2018). By far the highest concentrations of HCDs (mM range) in mammals are present in skeletal muscle (Gulewitsch and Amiradzibi 1900; Boldyrev et al. 2013) and receptor neurons of the olfactory epithelium (Margolis, 1974). Mammalian heart muscle also contains high concentration (mM range) of HCDs, though mainly as N-acetylated derivatives (O'Dowd et al. 1988). Much lower HCD concentrations are found in other tissues, with relatively high levels (upper µM range in mice) in brain and kidney (Boldyrev et al. 2013). Carnosine is degraded by cytosolic carnosinase CN2 (EC 3.4.13.18) and serum carnosinase CN1 (EC 3.4.13.20).
Numerous roles have been proposed for carnosine and other HCDs (Boldyrev et al. 2013). Carnosine has been shown to act as an anti-oxidant (Boldyrev et al. 1987; Kohen et al. 1988) and to inhibit the formation of advanced glycation and lipoxidation end products (Boldyrev et al. 2013). An anti-diabetic activity of carnosine has been documented in different animal models of type II diabetes (Sauerhöfer et al. 2007; Albrecht et al. 2017; Peters et al. 2018a; Artioli et al. 2019). A polymorphism of the CNDP1 gene resulting in a CN1 serum carnosinase with lower enzymatic activity is associated with reduced susceptibility for nephropathy in type II diabetes (Janssen et al. 2005). In line with this, carnosinase overexpression aggravates diabetes in diabetic mouse models (Sauerhöfer et al. 2007; Everaert et al. 2020; Qiu et al. 2020).
Carnosine deficient (Carns1−/−) mice (Wang-Eckhardt et al. 2020) and rats (de Souza Gonçalves et al. 2021) have been generated to further examine the physiological role of endogenous HCDs. These studies provided clear evidence for a role of carnosine in the maintenance of the olfactory epithelium in old and aging mice (Wang-Eckhardt et al. 2020) and in excitation–contraction coupling in rat cardiomyocytes (de Souza Gonçalves et al. 2021). In contrast, both studies could not identify an important role of carnosine and anserine in skeletal muscle. The present study was undertaken to further examine whether absence of endogenous carnosine synthesis increases protein oxidation and lipoxidation in old and aging mice, as suggested by the anti-oxidant function of administered carnosine. We focused on tissues with the highest HCD concentrations, i.e. skeletal muscle, heart, brain and kidney (Boldyrev et al. 2013).
Materials and methods
Carns1−/− and Leprdb/db mice and genotyping
Carns1−/− mice have been described previously (Wang-Eckhardt et al. 2020). Leptin receptor mutant Leprdb/+ mice (Coleman 1978) were obtained from Jackson Laboratories (stock number 000697). Carns1−/− mice were crossed with Leprdb/+ mice to obtain double deficient Leprdb/db/Carns1−/− mice. Genotyping of Carns1 was done as described (Wang-Eckhardt et al. 2020). Lepr genotyping was done by PCR using oligonucleotides 5′-AGA ACG GAC ACT CTT TGA AGT CTC-3′ and 5′-CAT TCA AAC CAT AGT TTA GGT TTG TGT-3′ followed by digestion with the restriction endonuclease RsaI, resulting in two fragments (108 bp and 27 bp) for the db allele and a 135 bp undigested PCR product for the wild-type allele. In the study using the Carns1 line, only males or females were compared in the individual experiments. However, for the quantitative analysis of all experiments data from males and females were combined. Forebrains were used for the analysis of brain tissues and musculus gastrocnemius for skeletal muscle.
Western blot analysis
Tissues were homogenized in 50 mM sodium phosphate (pH 7.4) with freshly added protease inhibitors (cOmplete protease inhibitor mix from Roche, Basel, Switzerland; or 1 × HALT from Thermo Fisher, Waltham, Massachusetts, USA). Protein concentrations were measured using the Biorad DC protein assay with bovine serum albumin as standard. SDS-PAGE (8%, 10% or 12.5% acrylamide, as indicated in the Figure legends) of proteins (10 or 20 µg/lane) was performed using acrylamide solution with a acrylamide:bisacrylamide ratio of 29:1 (cat# A515, Carl Roth, Karlsruhe, Germany) and the Mini-Protean Tetra cell gel electrophoresis system (Biorad, Hercules, California, USA), as described (Gallagher 2012). Semi-dry Western blot transfer onto nitrocellulose membranes (Amersham Protran 0.1 µm NC, cat# 10600000, GE Healthcare, Chicago, Illinois, USA) was performed using a Trans-Blot SD Semi-Dry Transfer Cell (Biorad) and standard procedures (Goldman et al. 2015). Protein marker used was PageRuler Prestained Protein Ladder (cat# 26617, Lot# 00395064, Thermo Fisher). Peroxidase labelled secondary antibodies were detected using Pierce ECL Western blotting substrate (Thermo Fisher) and a CCD camera (Fusion Solo with FusionCapt Advance Solo 4 software; Vilber Lourmat, Eberhardzell, Germany) and images were saved as TIFF files. Primary antibodies used: rabbit anti-Carns1 (Wang-Eckhardt et al. 2020; dilution 1:1000), rabbit anti-HNE (HNE11, Alpha Diagnostics, Reinach, Switzerland, Lot# 301718S31-P; dilution 1:4000), rabbit anti-MDA (MDA11, Alpha Diagnostics, Lot# 29111259-P; dilution 1:4000), mouse anti-GSR (sc-133245, Santa Cruz Biotechnology, Dallas, Texas, USA, Lot# C0519; dilution 1:2000), mouse anti-SOD1 (sc-101523, Santa Cruz, Lot# I0418; dilution 1:2000), mouse anti-SOD2 (sc-133134, Santa Cruz, Lot# A1520; dilution 1:2000), mouse anti-actin (A5316, Sigma-Aldrich, St. Louis, Missouri, USA, Lot# 018M4804V; dilution 1:10000), rabbit anti-α-tubulin (600-401-880, Rockland, Limerick, Pennsylvania, USA; dilution 1:20000). Secondary antibodies used: goat anti-rabbit peroxidase (111-035-003, Dianova, Hamburg, Germany; dilution 1:20000), goat anti-mouse peroxidase (115-035-044, Dianova; dilution 1:20000). Signal intensities of whole lanes or individual protein bands were determined by densitometry using the program AIDA image analysis software (Elysia-raytest, Straubenhardt, Germany) and TIFF files of Western blot images with signal intensities in the linear range.
Measurements in blood samples
All measurements in blood or plasma were performed using blood samples collected from the heart after mice have been killed by cervical dislocation.
Measurement of glycated haemoglobin: blood samples were mixed with four volumes of haemolysis puffer (150 mM NH4Cl, 1 mM KHCO3, 0.1 mM EDTA, pH 7.4) for five min, centrifuged at 3000×g for 10 min, supernatants removed and stored at -80 °C. Haemolysates were diluted in PBS and subjected to mouse GHbA1c ELISA (cat# E0709Mo; Bioassay Technology Laboratory, Shanghai, China) to determine glycated haemoglobin. Total haemoglobin was determined spectrophotometrically at 546 nm and the percentage of glycated haemoglobin was calculated.
Blood glucose determination: Fasting blood glucose (FBG) levels were determined after an overnight fasting period of 18 h using a blood glucose meter (Accu-Chek Aviva, Roche).
Plasma insulin determination: Plasma insulin was measured by ELISA (Ultra Sensitive Mouse Insulin ELISA Kit, cat# 90,080, Lot#09MYUMI081, Crystal Chem, Elk Grove Village, Iillinois, USA), following the manufacturer's instruction.
Quantification of protein carbonyl content
Protein carbonyl content in tissue homogenates (brain, kidney, skeletal muscle) was determined spectrophotometrically as described (Reznick and Packer 1994). Briefly, tissues were homogenized in 50 mM sodium phosphate (pH 7.4), containing complete protease inhibitor cocktail (Roche) and the supernatant (1 mL) obtained after centrifugation for 10 min at 6000×g was incubated with 10 mM 2,4-dinitrophenylhydrazine (DNPH) in 2.5 M HCl (4 mL) or only 2.5 M HCl (control) for one hour at room temperature (light protected). After precipitation with one volume of 20% (w/v) TCA, washing with 10% TCA and 3-times with ethanol/ethyl acetate (1:1, v/v), the protein pellet was dissolved in 6 M guanidine hydrochloride and absorbance was measured at 360 nm. Dinitrophenyl (DNP) content was calculated using an absorbance coefficient of 22,000 M−1 cm−1 (Reznick and Packer 1994). Protein content of homogenates was determined using DC protein assay (Bio-Rad). Western blot analysis of carbonylated proteins was done as described (Levine et al. 1994). Briefly, homogenates were mixed with one volume of 12% SDS, followed by two volumes of 20 mM DNPH in 10% trifluoroacetic acid (TFA) or 10% TFA for controls. After one hour reaction time, samples were neutralized by addition of 2 M Tris/30% glycerol and analysed by SDS-PAGE and Western blotting using rabbit anti-DNP antiserum (cat# D9656, Sigma-Aldrich, Lot# 110M4801; dilution 1:5000). Signal intensities were determined as described above in the Western blot analysis section.
Statistics
Data are shown as the mean ± standard deviation (SD) of at least three independent experiments (n = number of mice examined). Data were analysed using Excel (Microsoft, Richmond, Virginia, USA) or STATISTICA 6.0 (Statsoft, Tulsa, Oklahoma, USA). Data were tested for significant differences (p < 0.05) using one-way or two-way ANOVA with post hoc Tukey HSD test or two-tailed Student's t test.
Results
Carns1−/− mice do not have elevated levels of carbonylated proteins
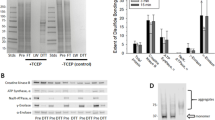
Carns1−/− mice used in this study lacked exons 3 to 9 and part of exon 10, i.e. all parts of the gene encoding amino acids of the Carns1 protein (Fig. 1A, B. We focused our analysis on old mice, as we expected it more likely that carnosine deficiency becomes noticeable in aging mice that are more prone to oxidative stress. A Kaplan–Meier plot showed normal survival rate of mice used for experiments of 18-month-old mice (Fig. 1C). Levels of protein-carbonylation in brain, skeletal muscle, kidney and heart were determined in 18-month-old mice by Western blot analysis after derivatization with DNPH (Fig. 2). Densitometric quantification of individual bands (see supplementary Figure S1) revealed higher intensities of single bands at about 50 kDa in kidney and 53 kDa in heart from Carns1−/− mice (arrow head in Fig. 2). We were, however, unable to decide whether this was caused by stronger carbonylation or increase in the concentration of a specific protein. There was an apparent tendency towards higher carbonyl content in skeletal muscle from Carns1−/− mice, however, we could not detect significantly increased DNP levels by spectrophotometry in skeletal muscle, as well as in brain and kidney (Table 1). Because of a low signal-to-noise ratio, we were unable to obtain reliable data for heart tissue by spectrophotometry; the Western blot analysis, however, suggested similar total DNP levels in both genotypes, besides the increase of protein carbonylation at 53 kDa (Fig. 2). In 24-month-old mice protein carbonyl content increased in brain (two-way ANOVA, effect of age: F1,11 = 55.18, p < 0.0001) and kidney (effect of age: F1,11 = 24.41; p < 0.001) compared to 18-month, but there was no significant effect of genotype (Table 1). Thus, we found no evidence that endogenous carnosine protects from increasing protein carbonylation in aging mice.
Carns1−/− mice. A Schematic presentation of the wild type Carns1 gene and the Carns1 knock-out allele in Carns1−/− mice analysed in this study. P/F indicate position of loxP and FRT sites that remain after cre mediated recombination in the knockout allele. All coding exons are deleted. B Western blot analysis (10% SDS-PAGE; 20 µg protein/lane) of skeletal muscle confirmed absence of Carns1 protein in Carns1−/− mice. C Kaplan–Meier curve showed no significant difference between genotypes in survival rate
Protein carbonylation in brain, skeletal muscle, kidney and heart from Carns1+/+ and Carns1−/− mice. A Tissue homogenates were treated with DNPH and analysed by 8% SDS-PAGE (10 µg protein/lane) and Western blotting using DNP specific antiserum. Age of mice was 18 months. Ponceau S staining served as loading control. In total, samples from at least 4 mice per genotype were examined. Representative experiments are shown. B Densitometric analysis showed that protein carbonyl bands at 50 kDa in kidney and 53 kDa in heart (labeled by arrow heads in A) had higher intensity in Carns1−/− mice. Data shown are mean ± SD (n = 4 mice per genotype). Quantification data for other protein bands are given in supplementary Fig. S1
Carns1−/− mice do not have elevated levels of advanced lipoxidation end products
To investigate to what extent endogenous carnosine may be essential for quenching lipid peroxidation products, levels of malondialdehyde (MDA) and 4-hydroxynonenal (HNE) protein adducts in brain, skeletal muscle, kidney, and heart were determined (Fig. 3). Densitometry of Western blots showed that there was neither a significant increase of individual MDA or HNE adduct bands (see supplementary Figs. S2 and S3) nor of the total amount of these adducts in Carns1−/− mice in any of the tissues examined. Additional experiments were done with younger mice (age of 7–8 months), which also showed no significant differences between genotypes (data not shown). We conclude that lipoxidation products were not increased in the absence of endogenous carnosine and related peptides, suggesting that there was no significant increase of oxidative stress in Carns1−/− mice.
Western blot analysis of MDA and HNE adducts in brain, kidney, heart and skeletal muscle from 18-month-old mice. Proteins (20 µg/lane) were separated by 10% SDS-PAGE. A MDA Western blots. B Quantification of MDA Western blots. C HNE Western blots. D Quantification of HNE Western blots. Shown are the mean ± SD; mean of Carns1+/+ set to 1 (n = 4 mice per genotype). Quantification data for individual protein bands are shown in supplementary Fig. S2 and S3
Anti-oxidant enzymes are not upregulated in Carns1−/− mice
Absence of elevated oxidative stress in Carns1−/− mice could be the result of a compensatory upregulation of anti-oxidant enzymes, e.g. superoxide dismutase, in response to oxidative stress (Miao and St. Clair 2009). We therefore examined levels of three anti-oxidant enzymes: glutathione reductase (GSR), superoxide dismutase 1 (SOD1, CuZnSOD) and superoxide dismutase 2 (SOD2, MnSOD) in brain, heart, kidney, and skeletal muscle (Fig. 4A). Mean levels of the three enzymes were not significantly altered in all tissues examined.
Western blot analysis of anti-oxidant enzymes. Proteins (10 µg/lane) were separated by 12.5% SDS-PAGE. A Representative Western blots of brain, heart, kidney and skeletal muscle from 18-month-old Carns1+/+ and Carns1−/− mice are shown. Blots were stained with antibodies against GSR, SOD1, SOD2 and tubulin. B Densitometric quantification of Western blots (normalized to tubulin). No significant differences were observed. Shown are the mean ± SD (n = 4 mice per genotype)
Absence of carnosine does not reinforce lipoxidation in kidney of Leprdb/db mice
Because an anti-lipoxidation effect of carnosine might be detectable only under pathological conditions associated with increased oxidative stress, we examined a diabetes mouse model (Leprdb/db) that is known to suffer from elevated oxidative stress and was expected to have higher levels of MDA in kidney, as suggested by higher levels of thiobarbituric acid reactive substances (DeRubertis et al. 2004). In line with this, MDA-adduct Western blot analysis revealed stronger signals in kidney extracts from 5-month-old Leprdb/db mice (Fig. 5A, B) compared to controls. In agreement with the experiments shown above (Fig. 3A, B), MDA-adducts were not increased in Carns1−/− mice compared to controls (Fig. 5A, B). Moreover, Carns1−/−/Leprdb/db mice did not show a further increase in MDA-adduct levels compared to Carns1+/+/Leprdb/db mice (Fig. 5B). Western blot analysis of GSR, SOD1, and SOD2 did not show significant differences between genotypes (Fig. 5C, D).
Analysis of MDA-adducts and anti-oxidant enzymes in Leprdb/db/Carns1−/− mice. A Western blot analysis (10% SDS-PAGE, 20 µg protein/lane) of MDA adducts in kidneys from Leprdb/db/Carns1−/− mice. B Densitometric quantification of MDA Western blots (mean ± SD, n = 4 mice per genotype). *p < 0.05; ns, not significant. C Western blot analysis (12.5% SDS-PAGE; 20 µg/lane) of anti-oxidant enzymes GSR, SOD1 and SOD2 in kidneys from 5-month-old mice (genotypes as indicated). D Densitometric quantification of GSR, SOD1 and SOD2 (mean ± SD, n = 4 mice per genotype). No significant differences were observed
Absence of carnosine does not affect body weight, blood glucose and insulin level in Leprdb/db mice
It has been shown that lower serum carnosine concentration in CN1 carnosinase-transgenic Leprdb/db mice correlates with higher fasting blood glucose (FBG) level (Sauerhöfer et al. 2007). No significant difference in FBG level was observed between Carns1−/− and Carns1+/+ mice (Fig. 6A). Glycated haemoglobin (Hb1Ac) concentration was also normal in Carns1−/− mice (Fig. 6B). As expected, Hb1Ac was increased in Leprdb/db/Carns1+/+ mice, but there was no further increase in Leprdb/db/Carns1−/− mice (Fig. 6C). We did not observe significant differences in FBG concentration in Leprdb/db/Carns1−/− compared to Leprdb/db/Carns1+/+ mice (Fig. 6D). The two genotypes did also not differ significantly with respect to insulin plasma concentration (Fig. 6E). Relative kidney weights of Leprdb/db mice were significantly reduced, but only as a consequence of the higher body weight (Fig. 6F, G). Neither absolute (not shown) nor relative kidney weights (Fig. 6G) differed significantly between Leprdb/db/Carns1+/+ and Leprdb/db/Carns1−/− mice. This is in line with results from Sauerhöfer et al. (2007) showing that renal hypertrophy in Leprdb/db mice does not correlate with carnosine concentration. In contrast to CN1-transgenic Leprdb/db mice (Sauerhöfer et al. 2007), Leprdb/db/Carns1−/− mice older than 15 weeks did not show progressive weight loss and body weights of 5-month-old Leprdb/db/Carns1+/+ and Leprdb/db/Carns1−/− mice were not significantly different (Fig. 6F). In summary, we found no evidence that Carns1 deficiency aggravates the phenotype of Leprdb/db mice.
FBG, insulin and Hb1Ac levels in Leprdb/db/Carns1−/− mice. A FBG was measured in male and female Carns1−/−, Carns1± and Carns1+/+ mice at 2, 3 and 18 months of age (n = number of animals examined). No significant differences were observed. B Hb1Ac levels in blood from 18-month-old female Carns1+/+ and Carns1−/− mice (n = 5 per genotype). No significant difference was found. C Hb1Ac level in blood from 5-month-old Carns1+/+ and Carns1−/− mice (n = 4 per genotype). D FBG level in 2-month- and 5-month-old mice of the genotypes indicated. For the non-diabetic mice data from Carns1−/−/Leprdb/+ and Carns1−/−/Lepr+/+ (2 months: n = 11; 5 months: n = 8), as well as Carns1+/+/Leprdb/+ and Carns1+/+/Lepr+/+ (2 months: n = 24; 5 months: n = 14) were combined (n = 6 for the other genotypes). E Insulin concentration in 5-month-old mice of the genotypes indicated (n = 8 for Carns1−/−/Leprdb/+ and Carns1+/+/Leprdb/+; n = 6 for Carns1−/−/Leprdb/+ and Carns1+/+/Leprdb/+. F Body weights at 5 months of age (n = 6 per genotype). G Relative kidney weights at 5 months of age (n = 6 per genotype). All data shown are the mean ± SD. Data were analysed by one-way ANOVA (except B: t test): *p < 0.05, **p < 0.001 (one-way ANOVA with post hoc Tukey HSD test)
Discussion
Several studies, reviewed by Boldyrev et al. (2013), have demonstrated that carnosine can reduce oxidative stress in vitro and in vivo. The anti-oxidant activity of carnosine is based on its reaction with superoxide anions (Kohen et al. 1991; Klebanov et al. 1997), reactive aldehydes like HNE and acrolein (Aldini et al. 2002; Baba et al. 2013; Zhao et al. 2019; Spaas et al. 2021) or carbonyl groups in oxidized proteins (Hipkiss et al. 2001). Carnosine may also increase activity of anti-oxidant enzymes, such as superoxide dismutase or glutathione peroxidase (Kim et al. 2011). Most studies have been performed by the administration of exogenous carnosine, which leaves the role of endogenous synthesized carnosine unresolved. In part this point has been addressed using transgenic serum carnosinase CN1 overexpression to reduce carnosine concentration (Sauerhöfer et al. 2007; Everaert et al. 2020; Qiu et al. 2020) and more recently mice and rats deficient in Carns1 (Wang-Eckhardt et al. 2020; de Souza Gonçalves et al. 2021) or GADL1, an enzyme involved in the synthesis of the carnosine precursor β-alanine (Mahootchi et al. 2020). While it can be assumed that Carns1−/− rodents lack HCDs in all tissues, CN1-transgene expression or carnosine/anserine supplementation primarily affect plasma and kidney ("circulating") HCD levels (Sauerhöfer et al. 2007; Qiu et al. 2019, 2020; Everaert et al. 2020, 2021). However, CN1-transgene expression also strongly reduced carnosine in brain (Qiu et al 2020), but not muscle (Everaert et al 2020). Thus, the use of CN1-transgene expression to explore the role of tissue carnosine/HCDs is limited.
Aging and several diseases, e.g. type 2 diabetes, are associated with elevated oxidative stress resulting in increased protein carbonyl content (Sohal et al. 1994; Tian et al. 1998; Moreno-Ulloa 2015; Hecker et al. 2018; Hauck et al. 2019). While we observed an age-dependent increase of protein carbonylation in aging mice and also elevated MDA protein adduct levels in Leprdb/db mice, absence of Carns1 apparently did not reinforce oxidative stress in aging or Leprdb/db mice. This finding suggests that in the analysed tissues other anti-oxidants play a more important role. Alternatively, other anti-oxidant systems may compensate loss of HCDs, though we could not find any evidence for an upregulation of SOD1 and SOD2, which are known to be induced in response to oxidative stress (Storz et al. 2005; Dell'Orco et al. 2016).
Potentially, absence of any significant effect on oxidative stress markers by Carns1-deficiency in contrast to models using carnosine supplementation or carnosinase overexpression could be a result of more efficient compensation by other systems in Carns1−/− mice, where HCDs are completely missing throughout development.
Although our results appear to contradict the assumed anti-oxidant role of carnosine, they are in line with several studies on the effect of supplemented carnosine on oxidative stress markers, which showed no or only weak effects on MDA or protein carbonyl content in rodents under non-pathological conditions (Aydin et al. 2010; Ozel Turkzu et al. 2010; Kalaz et al. 2014). These and other studies, however, indicated that a significant anti-oxidant activity of supplied carnosine may be observed in animal models exhibiting elevated oxidative stress (Ozel Turkzu et al. 2010; Kalaz et al. 2014; Xie et al. 2017; and references in Boldyrev et al. 2013). This appears to be the case also for HCDs synthesized in tissues, as ischemia–reperfusion injury in heart muscle and HNE and acrolein adduct formation are significantly reduced in Carns1-transgenic mice (Zhao et al. 2020). Whether Carns1−/− mice, however, are more susceptible towards increased oxidative stress has to be addressed in future studies.
Currently, there is evidence for a protective role of endogenously synthesized carnosine against oxidative stress only in the primary olfactory pathway, specifically the olfactory receptor neurons, which likely exhibit the highest carnosine concentration in rodent tissues (Kamal et al. 2009; Boldyrev et al. 2013). In Carns1−/− mice, increased levels of protein carbonylation, though not lipoxidation end products MDA and HNE, were found in the olfactory bulb (Wang-Eckhardt et al. 2020). An anti-oxidant role of carnosine in the primary olfactory pathway is further supported by the elevated levels of GSR in the olfactory bulb of glutamate decarboxylase like 1 (GADL1)-deficient mice, which have strongly reduced carnosine levels in the olfactory bulb (Mahootchi et al. 2020).
In line with the present study, no increased oxidative stress was observed in cardiomyocytes from CARNS1−/− rats (de Souza Gonçalves et al. 2021). In these rats, however, heart muscle showed impaired contractile function in combination with impaired calcium handling that indicated an important role of carnosine in excitation–contraction coupling, as also suggested for skeletal muscle in earlier studies (Dutka et al. 2012; Blancquaert et al. 2015).
There is an increased turnover of carnosine and increased activity of serum carnosinase CN1 in rodent models of diabetes and anti-diabetic properties of carnosine have been observed in several studies (Mong et al. 2011; Riedl et al. 2011; Ansurudeen et al. 2012; Peters et al. 2015, 2018b; Scuto et al. 2020). Type 2 diabetic patients have lower carnosine concentration in muscle (Gualano et al. 2012). Moreover, a polymorphism in the CNDP1 gene ("Mannheim allele") encoding serum carnosinase CN1 with reduced enzymatic activity is associated with a reduced risk of developing diabetic nephropathy (Janssen et al. 2005). Administration of carnosine increased insulin secretion and reduced FBG in two diabetes mouse models, Leprdb/db (Sauerhöfer et al. 2007) and BTBRob/ob mice (Albrecht et al. 2017). In addition, reduced serum carnosine levels correlated with increased FBG and reduced insulin concentration in CN1-transgenic Leprdb/db mice compared to non-transgenic Leprdb/db mice (Sauerhöfer et al. 2007). Interestingly, CN1-transgenic Leprdb/db mice showed massive glucosuria and significant weight loss at 15–20 weeks of age (Sauerhöfer et al. 2007). In contrast, we did not find significant differences in FBG, insulin and body weight in Carns1−/−/Leprdb/db compared to Carns1+/+/Leprdb/db mice. Thus, there is no absolute negative correlation between carnosine concentration and FBG level and insulin secretion under all experimental conditions, suggesting that insulin secretion is not directly influenced by carnosine. In line with this, carnosine at physiological concentrations was unable to stimulate insulin secretion in an insulinoma cell line (Sauerhöfer et al. 2007). In contrast to Carns1−/−/Leprdb/db mice, skeletal muscle in CN1-transgenic Leprdb/db mice can still release HCDs and high serum carnosinase activity likely leads to increased production of the hydrolysis products, β-alanine and histidine. If these differences may play a role to explain the apparently contradicting results obtained with the two animal models requires further studies.
In conclusion, we found no evidence to support the hypothesis that endogenously synthesized HCDs in mice are required to combat oxidative stress in those tissues that normally have the highest HCD concentrations (skeletal and heart muscle, brain and kidney). Thus, other anti-oxidant systems may play a more important role or can efficiently compensate loss of carnosine in mice. The possibility that endogenous HCDs may have a more important anti-oxidant role under pathological conditions associated with elevated oxidative stress remains to be examined.
Availability of data and material
Datasets generated in the current study are available from the corresponding author on reasonable request.
Code availability
Not applicable.
Abbreviations
- Carns1:
-
Carnosine synthase 1
- CN1:
-
Carnosinase 1
- CNDP1:
-
Carnosine dipeptidase 1
- DNP:
-
2,4-Dinitrophenol
- DNPH:
-
2,4-Dinitrophenylhydrazine
- FBG:
-
Fasting blood glucose
- GSR:
-
Glutathione reductase
- HCD:
-
Histidine-containing dipeptide
- HNE:
-
4-Hydroxynonenal
- Lepr:
-
Leptin receptor
- MDA:
-
Malondialdehyde
- SOD1:
-
Copper–zinc superoxide dismutase 1 (CuZnSOD)
- SOD2:
-
Manganese superoxide dismutase 2 (MnSOD)
References
Albrecht T, Schilperoort M, Zhang S, Braun JD, Qiu J, Rodriguez A, Pastene DO, Krämer BK, Köppel H, Baelde H, de Heer E, Anna Altomare A, Regazzoni L, Denisi A, Aldini G, van den Born J, Yard BA, Hauske SJ (2017) Carnosine attenuates the development of both type 2 diabetes and diabetic nephropathy in BTBR ob/ob mice. Sci Rep 7:44492. https://doi.org/10.1038/srep44492
Aldini G, Carini M, Beretta G, Bradamante S, Facino RM (2002) Carnosine is a quencher of 4-hydroxy-nonenal: through what mechanism of reaction? Biochem Biophys Res Commun 298:699–706. https://doi.org/10.1016/s0006-291x(02)02545-7
Ansurudeen I, Sunkari VG, Grünler J, Peters V, Schmitt CP, Catrina SB, Brismar K, Forsberg EA (2012) Carnosine enhances diabetic wound healing in the db/db mouse model of type 2 diabetes. Amino Acids 43:127–134. https://doi.org/10.1007/s00726-012-1269-z
Artioli GG, Sale C, Jones RL (2019) Carnosine in health and disease. Eur J Sport Sci 19:30–39. https://doi.org/10.1080/17461391.2018.1444096
Aydin AF, Küçükgergin C, Ozdemirler-Erata G, Koçak-Toker N, Uysal M (2010) The effect of carnosine treatment on prooxidant-antioxidant balance in liver, heart and brain tissues of male aged rats. Biogerontology 11:103–109. https://doi.org/10.1007/s10522-009-9232-4
Baba SP, Hoetker JD, Merchant M, Klein JB, Cai J, Barski OA, Conklin DJ, Bhatnagar A (2013) Role of aldose reductase in the metabolism and detoxification of carnosine-acrolein conjugates. J Biol Chem 288:28163–28179. https://doi.org/10.1074/jbc.M113.504753
Blancquaert L, Everaert I, Derave W (2015) Beta-alanine supplementation, muscle carnosine and exercise performance. Curr Opin Clin Nutr Metab Care 18:63–70. https://doi.org/10.1097/MCO.0000000000000127
Boldyrev AA, Dupin AM, Bunin AY, Babizhaev MA, Severin SE (1987) The antioxidative properties of carnosine, a natural histidine containing dipeptide. Biochem Int 15:1105–1113
Boldyrev AA, Aldini G, Derave W (2013) Physiology and pathophysiology of carnosine. Physiol Rev 93:1803–1845. https://doi.org/10.1152/physrev.00039.2012
Coleman DL (1978) Obese and diabetes: two mutant genes causing diabetes-obesity syndromes in mice. Diabetologia 14:141–148
Dell’Orco M, Milani P, Arrigoni L, Pansarasa O, Sardone V, Maffioli E, Polveraccio F, Bordoni M, Diamanti L, Ceroni M, Peverali FA, Tedeschi G, Cereda C (2016) Hydrogen peroxide-mediated induction of SOD1 gene transcription is independent from Nrf2 in a cellular model of neurodegeneration. Biochim Biophys Acta 1859:315–323. https://doi.org/10.1016/j.bbagrm.2015.11.009
DeRubertis FR, Craven PA, Melhem MF, Salah EM (2004) Attenuation of renal injury in db/db mice overexpressing superoxide dismutase: evidence for reduced superoxide-nitric oxide interaction. Diabetes 53:762–768
de Souza Gonçalves LS, Sales LP, Saito TR, Campos JC, Fernandes AL, Natali J, Jensen L, Arnold A, Ramalho L, Bechara LRG, Esteca MV, Correa I, Sant’Anna D, Ceroni A, Michelini LC, Gualano B, Teodoro W, Carvalho VH, Vargas BS, Medeiros MHG, Baptista IL, Irigoyen MC, Sale C, Ferreira JCB, Artioli GG (2021) Histidine dipeptides are key regulators of excitation-contraction coupling in cardiac muscle: evidence from a novel CARNS1 knockout rat model. Redox Biol 44:102016. https://doi.org/10.1016/j.redox.2021.102016
Drozak J, Veiga-da-Cunha M, Vertommen D, Stroobant V, Van Schaftingen E (2010) Molecular identification of carnosine synthase as ATP-grasp domain-containing protein 1 (ATPGD1). J Biol Chem 285:9346–9356. https://doi.org/10.1074/jbc.M109.095505
Dutka TL, Lamboley CR, McKenna MJ, Murphy RM, Lamb GD (2012) Effects of carnosine on contractile apparatus Ca2+ sensitivity and sarcoplasmic reticulum Ca2+ release in human skeletal muscle fibers. J Appl Physiol 112:728–736. https://doi.org/10.1152/japplphysiol.01331.2011
Everaert I, He J, Hanssens M, Stautemas J, Bakker K, Albrecht T, Zhang S, Van der Stede T, Vanhove K, Hoetker D, Howsam M, Tessier FJ, Yard B, Baba SP, Baelde HJ, Derave W (2020) Carnosinase-1 overexpression, but not aerobic exercise training, affects the development of diabetic nephropathy in BTBRob/ob mice. Am J Physiol Renal Physiol 318(4):F1030–F1040. https://doi.org/10.1152/ajprenal.00329.2019
Everaert I, Van der Stede T, Stautemas J, Hanssens M, van Aanhold C, Baelde H, Vanhaecke L, Derave W (2021) Oral anserine supplementation does not attenuate type-2 diabetes or diabetic nephropathy in BTBR ob/ob mice. Amino Acids 53:1269–1277. https://doi.org/10.1007/s00726-021-03033-4
Gallagher SR (2012) One-dimensional SDS gel electrophoresis of proteins. Curr Protoc Protein Sci Chapter 10:10.1.1-44. https://doi.org/10.1002/0471140864.ps1001s68
Goldman A, Ursitti JA, Mozdzanowski J, Speicher DW (2015) Electroblotting from Polyacrylamide Gels. Curr Protoc Protein Sci 82:10.7.1-10.7.16. https://doi.org/10.1002/0471140864.ps1007s82
Gualano B, Everaert I, Stegen S, Artioli GG, Taes Y, Roschel H, Achten E, Otaduy MC, Junior AH, Harris R, Derave W (2012) Reduced muscle carnosine content in type 2, but not in type 1 diabetic patients. Amino Acids 43:21–24. https://doi.org/10.1007/s00726-011-1165-y
Gulewitsch WS, Amiradzibi S (1900) Ueber das Carnosin, eine neue organische Base des Fleischextractes. Ber Dtsch Chem Ges 33:1902–1903
Hauck AK, Huang Y, Hertzel AV, Bernlohr DA (2019) Adipose oxidative stress and protein carbonylation. J Biol Chem 294:1083–1088. https://doi.org/10.1074/jbc.R118.003214
Hecker M, Wagner AH (2018) Role of protein carbonylation in diabetes. J Inherit Metab Dis 41:29–38. https://doi.org/10.1007/s10545-017-0104-9
Hipkiss AR, Brownson C, Carrier MJ (2001) Carnosine, the anti-ageing, anti-oxidant dipeptide, may react with protein carbonyl groups. Mech Ageing Dev 122:1431–1445. https://doi.org/10.1016/s0047-6374(01)00272-x
Janssen B, Hohenadel D, Brinkkoetter P, Peters V, Rind N, Fischer C, Rychlik I, Cerna M, Romzova M, de Heer E, Baelde H, Bakker SJ, Zirie M, Rondeau E, Mathieson P, Saleem MA, Meyer J, Köppel H, Sauerhoefer S, Bartram CR, Nawroth P, Hammes HP, Yard BA, Zschocke J, van der Woude FJ (2005) Carnosine as a protective factor in diabetic nephropathy: association with a leucine repeat of the carnosinase gene CNDP1. Diabetes 54:2320–2327. https://doi.org/10.2337/diabetes.54.8.2320
Kalaz EB, Çoban J, Aydın AF, Doğan-Ekici I, Doğru-Abbasoğlu S, Öztezcan S, Uysal M (2014) Carnosine and taurine treatments decreased oxidative stress and tissue damage induced by D-galactose in rat liver. J Physiol Biochem 70:15–25. https://doi.org/10.1007/s13105-013-0275-2
Kamal MA, Jiang H, Hu Y, Keep RF, Smith DE (2009) Influence of genetic knockout of Pept2 on the in vivo disposition of endogenous and exogenous carnosine in wild-type and Pept2 null mice. Am J Physiol Regul Integr Comp Physiol 296:R986–R991. https://doi.org/10.1152/ajpregu.90744.2008
Kim MY, Kim EJ, Kim YN, Choi C, Lee BH (2011) Effects of α-lipoic acid and L-carnosine supplementation on antioxidant activities and lipid profiles in rats. Nutr Res Pract 5:421–428. https://doi.org/10.4162/nrp.2011.5.5.421
Klebanov GI, Teselkin YuO, Babenkova IV, Popov IN, Levin G, Tyulina OV, Boldyrev AA, Vladimirov YuA (1997) Evidence for a direct interaction of superoxide anion radical with carnosine. Biochem Mol Biol Int 43:99–106. https://doi.org/10.1080/15216549700203861
Kohen R, Yamamoto Y, Cundy KC, Ames BN (1988) Antioxidant activity of carnosine, homocarnosine, and anserine present in muscle and brain. Proc Natl Acad Sci USA 85:3175–3179. https://doi.org/10.1073/pnas.85.9.3175
Kohen R, Misgav R, Ginsburg I (1991) The SOD like activity of copper:carnosine, copper:anserine and copper:homocarnosine complexes. Free Radic Res Commun 12–13:179–185. https://doi.org/10.3109/10715769109145784
Kwiatkowski S, Kiersztan A, Drozak J (2018) Biosynthesis of carnosine and related dipeptides in vertebrates. Curr Protein Pept Sci 19:771–789. https://doi.org/10.2174/1389203719666180226155657
Levine RL, Williams JA, Stadtman ER, Shacter E (1994) Carbonyl assays for determination of oxidatively modified proteins. Methods Enzymol 233:346–357. https://doi.org/10.1016/s0076-6879(94)33040-9
Mahootchi E, Cannon Homaei S, Kleppe R, Winge I, Hegvik TA, Megias-Perez R, Totland C, Mogavero F, Baumann A, Glennon JC, Miletic H, Kursula P, Haavik J (2020) GADL1 is a multifunctional decarboxylase with tissue-specific roles in β-alanine and carnosine production. Sci Adv 6:eabb3713. https://doi.org/10.1126/sciadv.abb3713
Margolis FL (1974) Carnosine in the primary olfactory pathway. Science 184:909–911. https://doi.org/10.1126/science.184.4139.909
Miao L, St Clair DK (2009) Regulation of superoxide dismutase genes: implications in disease. Free Radic Biol Med 47:344–356. https://doi.org/10.1016/j.freeradbiomed.2009.05.018
Mong MC, Chao CY, Yin MC (2011) Histidine and carnosine alleviated hepatic steatosis in mice consumed high saturated fat diet. Eur J Pharmacol 653:82–88. https://doi.org/10.1016/j.ejphar.2010.12.001
Moreno-Ulloa A, Nogueira L, Rodriguez A, Barboza J, Hogan MC, Ceballos G, Villarreal F, Ramirez-Sanchez I (2015) Recovery of indicators of mitochondrial biogenesis, oxidative stress, and aging with (-)-epicatechin in senile mice. J Gerontol A Biol Sci Med Sci 70:1370–1378. https://doi.org/10.1093/gerona/glu131
O’Dowd JJ, Robins DJ, Miller DJ (1988) Detection, characterisation, and quantification of carnosine and other histidyl derivatives in cardiac and skeletal muscle. Biochim Biophys Acta 967:241–249. https://doi.org/10.1016/0304-4165(88)90015-3
Ozel Turkcu U, Bilgihan A, Biberoglu G, Mertoglu Caglar O (2010) Carnosine supplementation protects rat brain tissue against ethanol-induced oxidative stress. Mol Cell Biochem 339:55–61. https://doi.org/10.1007/s11010-009-0369-x
Peters V, Lanthaler B, Amberger A, Fleming T, Forsberg E, Hecker M, Wagner AH, Yue WW, Hoffmann GF, Nawroth P, Zschocke J, Schmitt CP (2015) Carnosine metabolism in diabetes is altered by reactive metabolites. Amino Acids 47:2367–2376. https://doi.org/10.1007/s00726-015-2024-z
Peters V, Calabrese V, Forsberg E, Volk N, Fleming T, Baelde H, Weigand T, Thiel C, Trovato A, Scuto M, Modafferi S, Schmitt CP (2018a) Protective actions of anserine under diabetic conditions. Int J Mol Sci 19:2751. https://doi.org/10.3390/ijms19092751
Peters V, Zschocke J, Schmitt CP (2018b) Carnosinase, diabetes mellitus and the potential relevance of carnosinase deficiency. J Inherit Metab Dis 41:39–47. https://doi.org/10.1007/s10545-017-0099-2
Qiu J, Hauske SJ, Zhang S, Rodriguez-Niño A, Albrecht T, Pastene DO, van den Born J, van Goor H, Ruf S, Kohlmann M, Teufel M, Krämer BK, Hammes HP, Peters V, Yard BA, Kannt A (2019) Identification and characterisation of carnostatine (SAN9812), a potent and selective carnosinase (CN1) inhibitor with in vivo activity. Amino Acids 51:7–16. https://doi.org/10.1007/s00726-018-2601-z
Qiu J, Albrecht T, Zhang S, Hauske SJ, Rodriguez-Niño A, Zhang X, Nosan D, Pastene DO, Sticht C, Delatorre C, van Goor H, Porubsky S, Krämer BK, Yard BA (2020) Human carnosinase 1 overexpression aggravates diabetes and renal impairment in BTBROb/Ob mice. J Mol Med (berl) 98:1333–1346. https://doi.org/10.1007/s00109-020-01957-0
Reznick AZ, Packer L (1994) Oxidative damage to proteins: spectrophotometric method for carbonyl assay. Methods Enzymol 233:357–363. https://doi.org/10.1016/s0076-6879(94)33041-7
Riedl E, Pfister F, Braunagel M, Brinkkötter P, Sternik P, Deinzer M, Bakker SJ, Henning RH, van den Born J, Krämer BK, Navis G, Hammes HP, Yard B, Koeppel H (2011) Carnosine prevents apoptosis of glomerular cells and podocyte loss in STZ diabetic rats. Cell Physiol Biochem 28:279–288. https://doi.org/10.1159/000331740
Sauerhöfer S, Yuan G, Braun GS, Deinzer M, Neumaier M, Gretz N, Floege J, Kriz W, van der Woude F, Moeller MJ (2007) L-carnosine, a substrate of carnosinase-1, influences glucose metabolism. Diabetes 56:2425–2432. https://doi.org/10.2337/db07-0177
Scuto M, Trovato Salinaro A, Modafferi S, Polimeni A, Pfeffer T, Weigand T, Calabrese V, Schmitt CP, Peters V (2020) Carnosine activates cellular stress response in podocytes and reduces glycative and lipoperoxidative stress. Biomedicines 8:177. https://doi.org/10.3390/biomedicines8060177
Sohal RS, Ku HH, Agarwal S, Forster MJ, Lal H (1994) Oxidative damage, mitochondrial oxidant generation and antioxidant defenses during aging and in response to food restriction in the mouse. Mech Ageing Dev 74:121–133. https://doi.org/10.1016/0047-6374(94)90104-x
Spaas J, Franssen WMA, Keytsman C, Blancquaert L, Vanmierlo T, Bogie J, Broux B, Hellings N, van Horssen J, Posa DK, Hoetker D, Baba SP, Derave W, Eijnde BO (2021) Carnosine quenches the reactive carbonyl acrolein in the central nervous system and attenuates autoimmune neuroinflammation. J Neuroinflamm 18:255. https://doi.org/10.1186/s12974-021-02306-9
Storz P, Döppler H, Toker A (2005) Protein kinase D mediates mitochondrion-to-nucleus signaling and detoxification from mitochondrial reactive oxygen species. Mol Cell Biol 25:8520–8530. https://doi.org/10.1128/MCB.25.19.8520-8530.2005
Tian L, Cai Q, Wei H (1998) Alterations of antioxidant enzymes and oxidative damage to macromolecules in different organs of rats during aging. Free Radic Biol Med 24:1477–1484. https://doi.org/10.1016/s0891-5849(98)00025-2
Wang-Eckhardt L, Bastian A, Bruegmann T, Sasse P, Eckhardt M (2020) Carnosine synthase deficiency is compatible with normal skeletal muscle and olfactory function but causes reduced olfactory sensitivity in aging mice. J Biol Chem 295:17100–17113. https://doi.org/10.1074/jbc.RA120.014188
Xie RX, Li DW, Liu XC, Yang MF, Fang J, Sun BL, Zhang ZY, Yang XY (2017) Carnosine attenuates brain oxidative stress and apoptosis after intracerebral hemorrhage in rats. Neurochem Res 42:541–551. https://doi.org/10.1007/s11064-016-2104-9
Zhao J, Conklin DJ, Guo Y, Zhang X, Obal D, Guo L, Jagatheesan G, Katragadda K, He L, Yin X, Prodhan MAI, Shah J, Hoetker D, Kumar A, Kumar V, Wempe MF, Bhatnagar A, Baba SP (2020) Cardiospecific overexpression of ATPGD1 (Carnosine Synthase) increases histidine dipeptide levels and prevents myocardial ischemia reperfusion injury. J Am Heart Assoc 9:e015222. https://doi.org/10.1161/JAHA.119.015222
Zhao J, Posa DK, Kumar V, Hoetker D, Kumar A, Ganesan S, Riggs DW, Bhatnagar A, Wempe MF, Baba SP (2019) Carnosine protects cardiac myocytes against lipid peroxidation products. Amino Acids 51:123–138. https://doi.org/10.1007/s00726-018-2676-6
Funding
Open Access funding enabled and organized by Projekt DEAL. This work was supported by a grant of the Deutsche Forschungsgemeinschaft (grant number EC164/4–1 to ME). JY was supported by Hubei Chenguang Talented Youth Development Foundation.
Author information
Authors and Affiliations
Contributions
LWE, IB, YW, JY and ME performed experiments. LWE and ME supervised experimental work and analysed data. ME wrote the manuscript.
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no potential conflict of interest.
Ethics approval
Animal experiments were approved by the national authorities (Reference Number: 84–02.04.2012.A423).
Consent to participate
Not applicable.
Consent for publication
Not applicable.
Additional information
Handling editor: W. Derave.
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Wang-Eckhardt, L., Becker, I., Wang, Y. et al. Absence of endogenous carnosine synthesis does not increase protein carbonylation and advanced lipoxidation end products in brain, kidney or muscle. Amino Acids 54, 1013–1023 (2022). https://doi.org/10.1007/s00726-022-03150-8
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00726-022-03150-8