Abstract
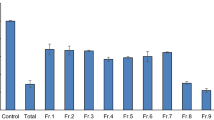
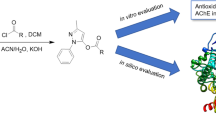
The use of acetylcholinesterase (AChE) inhibitors, antioxidants or multitarget compounds are among the main strategies against Alzheimer’s disease (AD). Between AChE inhibitors, those targeting the peripheral anionic site (PAS) are of special interest. Here, we describe the rational design and synthesis of peptide analogs of a natural PAS-targeting sequence that we recently discovered, aiming at increasing its activity against AChE. We also tested their radical scavenging and metal chelating properties. Our design strategy was based on the position-specific, computer-aided insertion of aromatic residues. The analog named as W3 showed a 30-fold higher inhibitory activity than the original sequence and an improved antioxidant activity. W3 is the most potent modified natural peptide against Electrophorus electricus AChE ever reported with an IC50 of 10.42 μM (± 1.02). In addition, it showed a radical scavenging activity of 47.00% ± 3.11 at 50 μM and 93.47% ± 1.53 at 400 μM. Since peptides are receiving increasing interest as drugs, we propose the W3 analog as an attractive sequence for the development of new peptide-based multitarget drugs for AD. Besides, this work sheds light on the importance of the aromatic residues in the modulation of AChE activity and their effect on the radical scavenging activity of a peptide.








Similar content being viewed by others
Data availability
Not applicable.
Code availability
Not applicable.
References
Agrawal H, Joshi R, Gupta M (2019) Purification, identification and characterization of two novel antioxidant peptides from finger millet (Eleusine coracana) protein hydrolysate. Food Res Int 120:697–707. https://doi.org/10.1016/j.foodres.2018.11.028
Akhoon BA, Choudhary S, Tiwari H, Kumar A, Barik MR, Rathor L, Pandey R, Nargotra A (2020) Discovery of a new donepezil-like acetylcholinesterase inhibitor for targeting Alzheimer’s disease: computational studies with biological validation. J Chem Inf Model 60(10):4717–4729. https://doi.org/10.1021/acs.jcim.0c00496
Albertini C, Salerno A, de Sena Murteira Pinheiro P, Bolognesi ML (2020) From combinations to multitarget-directed ligands: a continuum in Alzheimer’s disease polypharmacology. Med Res Rev 41:2606–2633. https://doi.org/10.1002/med.21699
Case DA, Cheatham TE III, Darden T, Gohlke H, Luo R, Merz KM Jr, Onufriev A, Simmerling C, Wang B, Woods R (2005) The Amber biomolecular simulation programs. J Computat Chem 26:1668–1688. https://doi.org/10.1002/jcc.20290
Chen Z, Zhong C (2014) Oxidative stress in Alzheimer’s disease. Neurosci Bull 30:271–281. https://doi.org/10.1007/s12264-013-1423-y
Chen X, Wehle S, Kuzmanovic N, Merget B, Holzgrabe U, König B, Sotriffer CA, Decker M (2014) Acetylcholinesterase inhibitors with photoswitchable inhibition of β-amyloid aggregation. ACS Chem Neurosci 5:377–389. https://doi.org/10.1021/cn500016p
Cockroft SL, Hunter CA, Lawson KR, Perkins J, Urch CJ (2005) Electrostatic control of aromatic stacking interactions. J Am Chem Soc 127:8594–8595. https://doi.org/10.1021/ja050880n
Copeland RA (2005) Evaluation of enzyme inhibitors in drug discovery: a guide for medicinal chemists and pharmacologists, 2nd edn. Wiley, Hoboken
Cummings J, Lee G, Zhong K, Fonseca J, Taghva K (2021) Alzheimer’s disease drug development pipeline: 2021. Alzheimer’s Dement. https://doi.org/10.1002/trc2.12179
Dominguez C, Boelens R, Bonvin AM (2003) HADDOCK: a protein-protein docking approach based on biochemical and/or biophysical information. J Am Chem Soc 125:1731–1737. https://doi.org/10.1021/ja026939x
dos Santos TC, Gomes TM, Serra Pinto BA, Camara AL, de Andrade Paes AM (2018) Naturally occurring acetylcholinesterase inhibitors and their potential use for Alzheimer’s disease therapy. Front Pharmacol 9:1192. https://doi.org/10.3389/fphar.2018.01192
El-Hayek YH, Wiley RE, Khoury CP, Daya RP, Ballard C, Evans AR, Karran M, Molinuevo JL, Norton M, Atri A (2019) Tip of the iceberg: assessing the global socioeconomic costs of Alzheimer’s disease and related dementias and strategic implications for stakeholders. J Alzheimers Dis 70:323–341. https://doi.org/10.3233/JAD-190426
Ellman GL, Courtney KD, Andres VJR, Feather-Stone RM (1961) A new and rapid colorimetric determination of acetylcholinesterase activity. Biochem Pharmacol 7:88–95. https://doi.org/10.1016/0006-2952(61)90145-9
Eyer P, Worek F, Kiderlen D, Sinko G, Stuglin A, Simeon-Rudolf V, Reiner E (2003) Molar absorption coefficients for the reduced Ellman reagent: reassessment. Anal Biochem 312:224–227. https://doi.org/10.1016/s0003-2697(02)00506-7
Fosgerau K, Hoffmann T (2015) Peptide therapeutics: current status and future directions. Drug Discov Today 20:122–128. https://doi.org/10.1016/j.drudis.2014.10.003
Francis PT, Ramírez MJ, Lai MK (2010) Neurochemical basis for symptomatic treatment of Alzheimer’s disease. Neuropharmacology 59:221–229. https://doi.org/10.1016/j.neuropharm.2010.02.010
Götz AW, Williamson MJ, Xu D, Poole D, Le Grand S, Walker RC (2012) Routine microsecond molecular dynamics simulations with AMBER on GPUs. 1. Generalized born. J Chem Theory Comput 8(5):1542–1555. https://doi.org/10.1021/ct20009j
Hansen PR (2016) Antimicrobial peptides: methods and protocols, 1st edn. Humana Press, New York
Harel M, Schalk I, Ehret-Sabatier L, Bouet F, Goeldner M, Hirth C, Axelsen PH, Silman I, Sussman JL (1993) Quaternary ligand binding to aromatic residues in the active-site gorge of acetylcholinesterase. Proc Natl Acad Sci USA 90:9031–9035. https://doi.org/10.1073/pnas.90.19.9031
Harel M, Kleywegt GJ, Ravelli RBG, Silman I, Sussman JL (1995) Crystal structure of an acetylcholinesterase–fasciculin complex: interaction of a three-fingered toxin from snake venom with its target. Structure 3:1355–1366. https://doi.org/10.1016/s0969-2126(01)00273-8
Hartigan A, Wong MA (1979) A K-means clustering algorithm. J R Stat Soc 28(1):100–108. https://doi.org/10.2307/2346830
Hunter CA, Lawson KR, Perkins J, Urch CJ (2001) Aromatic interactions. J Chem Soc Perkin Trans 2:651–669. https://doi.org/10.1039/B008495F
Ibrahim MM, Gabr MT (2019) Multitarget therapeutic strategies for Alzheimer’s disease. Neural Regen Res 14:437–440. https://doi.org/10.4103/1673-5374.245463
Johnson G, Moore SW (2006) The peripheral anionic site of acetylcholinesterase: structure, functions and potential role in rational drug design. Curr Pharm Des 12:217–225. https://doi.org/10.2174/138161206775193127
Ko SC, Kim D, Jeon YJ (2012) Protective effect of a novel antioxidative peptide purified from a marine Chlorella ellipsoidea protein against free radical-induced oxidative stress. Food Chem Toxicol 50:2294–2302. https://doi.org/10.1016/j.fct.2012.04.022
Kryger G, Silman I, Sussman JL (1999) Structure of acetylcholinesterase complexed with E2020 (Aricept): implications for the design of new anti-Alzheimer drugs. Structure 15:297–307. https://doi.org/10.1016/s0969-2126(99)80040-9
Kuhn-Nentwig L, Sheynis T, Kolusheva S, Nentwig W, Jelinek R (2013) N-terminal aromatic residues closely impact the cytolytic activity of cupiennin 1a, a major spider venom peptide. Toxicon 75:177–186. https://doi.org/10.1016/j.toxicon.2013.03.003
Lakey-Beitia J, Burillo AM, La Penna G, Hegde ML, Raof KS (2021) Polyphenols as potential metal chelation compounds against Alzheimer’s disease. J Alzheimers Dis 82(Suppl 1):S335–S357. https://doi.org/10.3233/JAD-200185
Lau JL, Dunn MK (2018) Therapeutic peptides: Historical perspectives, current development trends, and future directions. Bioorg Med Chem 26:2700–2707. https://doi.org/10.1016/j.bmc.2017.06.052
Li Q, Shi C, Wang M, Zhou M, Liang M, Zhang T, Yuan E, Wang Z, Yao M, Ren J (2019) Tryptophan residue enhances in vitro walnut protein-derived peptides exerting xanthine oxidase inhibition and antioxidant activities. J Funct Foods 53:276–285. https://doi.org/10.1016/j.jff.2018.11.024
Maier JA, Martinez C, Kasavajhala K, Wickstrom L, Hauser KE, Simmerling C (2015) ff14SB: improving the accuracy of protein side chain and backbone parameters from ff99SB. J Chem Theory Comput 11:3696–3713. https://doi.org/10.1021/acs.jctc.5b00255
Masters CL, Bateman R, Blennow K, Rowe CC, Sperling RA, Cummings JL (2015) Alzheimer’s disease. Nat Rev Dis Primers 1:15056. https://doi.org/10.1038/nrdp.2015.56
Matsui R, Honda R, Kanome M, Hagiwara A, Matsuda Y, Togitani T, Ikemoto N, Terashima M (2018) Designing antioxidant peptides based on the antioxidant properties of the amino acid side-chains. Food Chem 245:750–755. https://doi.org/10.1016/j.foodchem.2017.11.119
Memarpoor-Yazdi M, Mahaki H, Zare-Zardini H (2013) Antioxidant activity of protein hydrolysates and purified peptides from Zizyphus jujuba fruits. J Funct Foods 5:62–70. https://doi.org/10.1016/j.jff.2012.08.004
Mirzaei M, Mirdamadi S, Safavi M, Zare D, Hadizadeh M, Asadi MM (2019) Synthesis, in vitro and cellular antioxidant activity evaluation of novel peptides derived from Saccharomyces cerevisiae protein hydrolysate: structure–function relationship. Amino Acids 51:1167–1175. https://doi.org/10.1007/s00726-019-02752-z
Muttenthaler M, King GF, Adams DJ, Alewood PF (2021) Trends in peptide drug discovery. Nat Rev Drug Discov 20(4):309–325. https://doi.org/10.1038/s41573-020-00135-8
Nabil ZI, Hussein AA, Zalat SM, Rakha MKh (1998) Mechanism of action of honey bee (Apis mellifera L.) venom on different types of muscles. Hum Exp Toxicol 17:185–190. https://doi.org/10.1177/096032719801700311
Nayak BN, Buttar HS (2016) Evaluation of antioxidant properties of tryptophan and its metabolites in In vitro assay. J Complement Integr Med 13:129–136. https://doi.org/10.1515/jcim-2015-0051
Pohanka M (2011) Cholinesterases, a target of pharmacology and toxicology. Biomed Pap Med Fac Univ Palacky Olomouc Czech Repub 155:219–229. https://doi.org/10.5507/bp.2011.036
Rastelli G, Del Rio A, Degliesposti G, Sgobba M (2010) Fast and accurate predictions of binding free energies using MM-PBSA and MM-GBSA. J Comput Chem 31:797–810. https://doi.org/10.1002/jcc.21372
Raves ML, Harel M, Pang YP, Silman I, Kozikowski AP, Sussman JL (1997) Structure of acetylcholinesterase complexed with the nootropic alkaloid, (-)-huperzine A. Nat Struct Mol Biol 4:57–63. https://doi.org/10.1038/nsb0197-57
Rodríguez-Ithurralde D, Silveira R, Barbeito L, Dajas F (1983) Fasciculin, a powerful anticholinesterase polypeptide from Dendroaspis angusticeps venom. Neurochem Int 5:267–274. https://doi.org/10.1016/0197-0186(83)90028-1
Sadeghi L, Yekta R, Dehghan G (2020) The inhibitory effects of bile acids on catalytic and non-catalytic functions of acetylcholinesterase as a therapeutic target in Alzheimer’s disease. Acta Neurobiol Exp 80:108–116. https://doi.org/10.21307/ane-2020-011
Sakano T, Mahamood MdI, Yamashita T, Fujitani H (2016) Molecular dynamics analysis to evaluate docking pose prediction. Biophys Physicobiol 13:181–194. https://doi.org/10.2142/biophysico.13.0_181
Salomon-Ferrer R, Götz AW, Poole D, Le Grand S, Walker RC (2013) Routine microsecond molecular dynamics simulations with AMBER on GPUs. 2. explicit solvent particle Mesh Ewald. J Chem Theory Comput 9(9):3878–3888. https://doi.org/10.1021/ct400314y
Sanchis I, Spinelli R, Aschemacher N, Humpola MV, Siano A (2020) Acetylcholinesterase inhibitory activity of a naturally occurring peptide isolated from Boana pulchella (Anura: Hylidae) and its analogs. Amino Acids 52:387–396. https://doi.org/10.1007/s00726-019-02815-1
Sepsova V, Karasova JZ, Korabecny J, Dolezal R, Zemek F, Bennion BJ, Kuca K (2013) Oximes: inhibitors of human recombinant acetylcholinesterase. A structure-activity relationship (SAR) Study. Int J Mol Sci 14:16882–16900. https://doi.org/10.3390/ijms140816882
Shen Y, Maupetit J, Derreumaux P, Tufféry P (2014) Improved PEPFOLD approach for peptide and miniprotein structure prediction. J Chem Theory Comput 10:4745–4758. https://doi.org/10.1021/ct500592m
Siano A, Garibotto FF, Andujar SA, Baldoni HA, Tonarelli GG, Enriz RD (2017) Molecular design and synthesis of novel peptides from amphibians skin acting as inhibitors of cholinesterase enzymes. J Pept Sci 23:236–244. https://doi.org/10.1002/psc.2974
Sinnokrot MO, Sherrill CD (2003) Unexpected substituent effects in face-to-face π-stacking interactions. J Phys Chem A 107:8377–8379. https://doi.org/10.1021/jp030880e
Spinelli R, Sanchis I, Aimaretti FM, Attademo AM, Portela M, Humpola MV, Tonarelli GG, Siano AS (2019) Natural Multi-target inhibitors of cholinesterases and monoamine oxidase enzymes with antioxidant potential from skin extracts of Hypsiboas cordobae and Pseudis minuta (Anura: Hylidae). Chem Biodivers 16:e1800472. https://doi.org/10.1002/cbdv.201800472
Spinelli R, Aimaretti FM, López JA, Siano AS (2021) Amphibian skin extracts as source of bioactive multi-target agents against different pathways of Alzheimer’s disease. Nat Prod Res 35(4):686–689. https://doi.org/10.1080/14786419.2019.1591396
Strelow J, Dewe W, Iversen PW, Brooks HB, Radding JA, McGee J, Weidner J (2004) Mechanism of action assays for enzymes. In: Markossian S, Sittampalam GS, Grossman A (eds) Assay guidance manual. Eli Lilly & Company and the National Center for Advancing Translational Sciences, Bethesda, pp 120–121
Sugimoto H, Yamanishi Y, Ogura H, Iimura Y, Yamatsu K (1999) Discovery and development of donepezil hydrochloride for the treatment of Alzheimer’s disease. Yakugaku Zasshi 119:101–113. https://doi.org/10.1248/yakushi1947.119.2_101
Tanzadehpanah H, Asoodeh A, Saidijam M, Chamani J, Mahaki H (2018) Improving efficiency of an angiotensin converting enzyme inhibitory peptide as multifunctional peptides. J Biomol Struct Dyn 36:3803–3818. https://doi.org/10.1080/07391102.2017.1401001
Thévenet P, Shen Y, Maupetit J, Guyon F, Derreumaux P, Tufféry P (2012) PEP-FOLD: an updated de novo structure prediction server for both linear and disulfide bonded cyclic peptides. Nucleic Acids Res 40:288–293. https://doi.org/10.1093/nar/gks419
Tranches Dias KS, Viegas C Jr (2014) Multi-target directed drugs: a modern approach for design of new drugs for the treatment of Alzheimer’s disease. Curr Neuropharmacol 12:239–255. https://doi.org/10.2174/1570159X1203140511153200
Tsui V, Case DA (2000) Theory and applications of the generalized Born solvation model in macromolecular simulations. Biopolymers 56:275–291. https://doi.org/10.1002/1097-0282(2000)56:4%3c275::AID-BIP10024%3e3.0.CO;2-E
van Zundert GCP, Rodrigues JPGLM, Trellet M, Schmitz C, Kastritis PL, Karaca E, Melquiond ASJ, van Dijk M, de Vries SJ, Bonvin AMJJ (2016) The HADDOCK2.2 Web server: user-friendly integrative modeling of biomolecular complexes. J Mol Biol 428:720–725. https://doi.org/10.1016/j.jmb.2015.09.014
Wang G, Li X, Wang Z (2016a) APD3: the antimicrobial peptide database as a tool for research and education. Nucleic Acids Res 44:1087–1093. https://doi.org/10.1093/nar/gkv1278
Wang L, Chen L, Yu M, Xu LH, Cheng B, Lin YS, Gu Q, He XH, Xu J (2016b) Discovering new mTOR inhibitors for cancer treatment through virtual screening methods and in vitro assays. Sci Rep 6:18987. https://doi.org/10.1038/srep18987
Yaneva SA, Stoykova II, Ilieva LI, Vezenkov LT, Marinkova DA, Yotova LK, Raykova RN, Danalev DL (2017) Acetylcholinesterase inhibition activity of peptide analogues of galanthamine with potential application for treatment of Alzheimer`s disease. Bulg Chem Commun 49:90–94
Yu Z, Wu S, Zhao W, Ding L, Fan Y, Shiuan D, Liu J, Chen F (2018) Anti-Alzheimers activity and molecular mechanism of albumin-derived peptides against AChE and BchE. Food Funct 9:1173–1178. https://doi.org/10.1039/c7fo01462g
Zhu CZ, Zhang WG, Zhou GH, Xu XL (2016) Identification of antioxidant peptides of Jinhua ham generated in the products and through the simulated gastrointestinal digestion system. J Sci Food Agric 96:99–108. https://doi.org/10.1002/jsfa.7065
Zoete V, Irving MB, Michielin O (2010) MM-GBSA binding free energy decomposition and T cell receptor engineering. J Mol Recognit 23:142–152. https://doi.org/10.1002/jmr.1005
Funding
This work was financially supported by the Argentinian National Scientific and Technical Research Council (CONICET) of the Ministry of Science, Technology and Innovation under grants PICT-2017-0035, PIP 2017-2019 GI. 112 201701 00462 CO.
Author information
Authors and Affiliations
Contributions
IS and AS: Conceptualization; IS, RS, and NA: Methodology; investigation and result analysis: IS (Synthesis, in vitro assays, computational studies), RS (in vitro assays), NA (Synthesis); IS: Writing—original draft preparation; IS, RS, NA, and AS: Writing—review and editing; AS: Funding acquisition; AS: Supervision.
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Additional information
Handling editor: K. Aoyama.
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Sanchis, I., Spinelli, R., Aschemacher, N. et al. Rational design and synthesis of modified natural peptides from Boana pulchella (anura) as acetylcholinesterase inhibitors and antioxidants. Amino Acids 54, 181–192 (2022). https://doi.org/10.1007/s00726-021-03096-3
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00726-021-03096-3