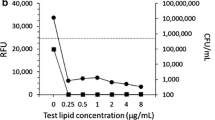
Abstract
Antimicrobial peptides (AMPs) have the ability to penetrate as well as transport cargo across bacterial cell membranes, and they have been labeled as exceptional candidates to function in drug delivery. The aim of this study was to investigate the effectiveness of novel formulation of AMPs for enhanced MRSA activity. The strategy was carried out through the formulation of liposomes by thin-layer film hydration methodology, containing phosphatidylcholine, cholesterol, oleic acid, the novel AMP, as well as vancomycin (VCM). Characterization of the AMPs and liposomes included HPLC and LCMS for peptide purity and mass determination; DLS (size, polydispersity, zeta potential), TEM (surface morphology), dialysis (drug release), broth dilution, and flow cytometry (antibacterial activity); MTT assay, haemolysis and intracellular antibacterial studies. The size, PDI, and zeta potential of the drug-loaded AMP2-Lipo-1 were 102.6 ± 1.81 nm, 0.157 ± 0.01, and − 9.81 ± 1.69 mV, respectively, while for AMP3-Lipo-2 drug-loaded formulation, it was 146.4 ± 1.90 nm, 0.412 ± 0.05, and − 4.27 ± 1.25 mV respectively at pH 7.4. However, in acidic pH for both formulations, we observed an increase in size, PDI, and a switch to positive zeta potential, which indicated the pH responsiveness of our liposomal systems. The in vitro drug release studies demonstrated that liposomal formulations released VCM-HCl at a faster rate at pH 6.0 compared to pH 7.4. In vitro antibacterial activity against S. aureus and MRSA revealed that liposomes had enhanced activity at pH 6 compared to pH 7.4. The study revealed that the formulation can potentially be used to enhance activity and penetration of AMPs, thereby improving the treatment of bacterial infections.










Similar content being viewed by others
References
Abraham MJ, Murtola T, Schulz R, Páll S, Smith JC, Hess B, Lindahl E (2015) GROMACS: High performance molecular simulations through multi-level parallelism from laptops to supercomputers. SoftwareX 1–2:19–25
Abu Lila AS, Ishida T (2017) Liposomal delivery systems: design optimization and current applications. Biol Pharm Bull 40:1–10
Akbarzadeh A, Rezaei-Sadabady R, Davaran S, Joo SW, Zarghami N, Hanifehpour Y, Samiei M, Kouhi M, Nejati-Koshki K (2013) Liposome: classification, preparation, and applications. Nanoscale Res Lett 8:102
Alves ID, Goasdoué N, Correia I, Aubry S, Galanth C, Sagan S, Lavielle S, Chassaing G (2008) Membrane interaction and perturbation mechanisms induced by two cationic cell penetrating peptides with distinct charge distribution. Biochim Biophys Acta Gen Subj 1780:948–959
Antimicrobial cell penetrating peptides with bacterial cell specificity (2018) Pharmacophore modeling, quantitative structure activity relationship and molecular dynamics simulation. J Biomol Struct Dyn 1:153–173
Arias M, Piga KB, Hyndman ME, Vogel HJ (2018) Improving the activity of Trp-rich antimicrobial peptides by Arg/Lys Substitutions and changing the length of cationic residues. Biomolecules 8:19
Bahnsen JS, Franzyk H, Sandberg-Schaal A, Nielsen HM (1828) Antimicrobial and cell-penetrating properties of penetratin analogs: Effect of sequence and secondary structure. Biochim Biophys Acta Biomembr 2013:223–232
Biswaro LS, Sousa MG, Rezende TMB, Dias SC, Franco OL (2018) Antimicrobial peptides and nanotechnology, recent advances and challenges. Front Microbiol 9:855
Bozzuto G, Molinari A (2015) Liposomes as nanomedical devices. Int J Nanomed 10:975
Brauner A, Fridman O, Gefen O, Balaban NQ (2016) Distinguishing between resistance, tolerance and persistence to antibiotic treatment. Nat Rev Microbiol 14:320
Brinch KS, Tulkens PM, Van Bambeke F, Frimodt-Møller N, Høiby N, Kristensen H-H (2010) Intracellular activity of the peptide antibiotic NZ2114: studies with Staphylococcus aureus and human THP-1 monocytes, and comparison with daptomycin and vancomycin. J Antimicrob Chemother 65:1720–1724
Bulbake U, Doppalapudi S, Kommineni N, Khan W (2017) Liposomal formulations in clinical use: an updated review. Pharmaceutics 9:12
Bussi G, Donadio D, Parrinello M (2007) Canonical sampling through velocity rescaling. J Chem Phys 126:14101
Caldeira S, de Araújo Lopes M, Novais VM, Salviano Teixeira C, Honorato-Sampaio K, Tadeu Pereira M, Ferreira LAM, Braga FC, Cristina Oliveira M (2013) Preparation, physicochemical characterization, and cell viability evaluation of long-circulating and pH-sensitive liposomes containing ursolic acid. Biomed Res Int 13:190–345
Cao B, Christophersen L, Thomsen K, Sønderholm M, Bjarnsholt T, Jensen PØ, Høiby N, Moser C (2015) Antibiotic penetration and bacterial killing in a Pseudomonas aeruginosa biofilm model. J Antimicrob Chemother 70:2057–2063
Carmona-Ribeiro AM, de Melo Carrasco LD (2014) Novel formulations for antimicrobial peptides. Int J Mol Sci 15:18040–18083
Chambers HF, DeLeo FR (2009) Waves of resistance: Staphylococcus aureus in the Antibiotic Era. Nat Rev Microbiol 7:629–641
Chatin B, Mével M, Devallière J, Dallet L, Haudebourg T, Peuziat P, Colombani T, Berchel M, Lambert O, Edelman A, Pitard B (2015) Liposome-based formulation for intracellular delivery of functional proteins. Mol Ther Nucleic Acids 4:e244
Chiang C-Y, Uzoma I, Moore RT, Gilbert M, Duplantier AJ, Panchal RG (2018) Mitigating the impact of antibacterial drug resistance through host-directed therapies: current progress, outlook, and challenges. Mbio 9:18
Close WL, Glassbrook JE, Gurczynski SJ, Pellett PE (2018) Infection-induced changes within the endocytic recycling compartment suggest a roadmap of human cytomegalovirus egress. Front. Microbiol 9:2
de Jong DH, Singh G, Bennett WFD, Arnarez C, Wassenaar TA, Schafer LV, Periole X, Tieleman DP, Marrink SJ (2013) Improved parameters for the martini coarse-grained protein force field. J Chem Theory Comput 9:687–697
Desai P, Patlolla RR, Singh M (2010) Interaction of nanoparticles and cell-penetrating peptides with skin for transdermal drug delivery. Mol Membr Biol 27:247–259
Deshpande PP, Biswas S, Torchilin VP (2013) Current trends in the use of liposomes for tumor targeting. Nanomedicine 8:1509–1528
Epand RM, Epand RF (2009) Lipid domains in bacterial membranes and the action of antimicrobial agents. Biochim Biophys Acta Biomembr 1788:289–294
Epand RM, Walker C, Epand RF, Magarvey NA (1858) Molecular mechanisms of membrane targeting antibiotics. Biochim Biophys Acta Biomembr 2016:980–987
Exl L, Bance S, Reichel F, Schrefl T, Peter Stimming H, Mauser NJ (2014) LaBonte’s method revisited: an effective steepest descent method for micromagnetic energy minimization. J Appl Phys 115:17D118
Gautam A, Chaudhary K, Kumar R, Sharma A, Kapoor P, Tyagi A, Raghava GPS (2013) In silico approaches for designing highly effective cell penetrating peptides. J Transl Med 11:74
Gautam A, Chaudhary K, Kumar R, Raghava GPS (2015) Computer-aided virtual screening and designing of cell-penetrating peptides. Springer, London
Grant SS, Hung DT (2013) Persistent bacterial infections, antibiotic tolerance, and the oxidative stress response. Virulence 4:273–283
Han J, Huang Y, Chen X, Zhou F, Fei Y, Fu J (2018) Lipidation and conformational constraining for prolonging the effects of peptides: Xenopus glucagon-like peptide 1 analogues with potent and long-acting hypoglycemic activity. Eur J Pharm Sci 123:111–123
Hansen A, Schäfer I, Knappe D, Seibel P, Hoffmann R (2012) Intracellular toxicity of proline-rich antimicrobial peptides shuttled into mammalian cells by the cell-penetrating peptide penetratin. Antimicrob Agents Chem 56:5194–5201
Heidarli E, Dadashzadeh S, Haeri A (2017) State of the art of stimuli-responsive liposomes for cancer therapy. Iran J Pharm Res IJPR 16:1273–1304
Hendricks MP, Sato K, Palmer LC, Stupp SI (2017) Supramolecular assembly of peptide. Amphiphiles 1:2440–2448
Hsu C-Y, Yang S-C, Sung CT, Weng Y-H, Fang J-Y (2017) Anti-MRSA malleable liposomes carrying chloramphenicol for ameliorating hair follicle targeting. Int J Nanomed 12:8227–8238
Hu X, Zhang Y, Xie Z, Jing X, Bellotti A, Gu Z (2017) Stimuli-responsive polymersomes for biomedical applications. Biomacromol 18:649–673
Irazazabal LN, Porto WF, Ribeiro SM, Casale S, Humblot V, Ladram A, Franco OL (1858) Selective amino acid substitution reduces cytotoxicity of the antimicrobial peptide mastoparan. Biochim Biophys Acta Biomembr 2016:2699–2708
Jo S, Cheng X, Lee J, Kim S, Park S-J, Patel DS, Beaven AH, Rui KH, Park S, Lee HS, Roux B, MacKerell AD Jr, Klauda JB, Qi Y, Im W (2017) CHARMM-GUI 10 years for biomolecular modeling and simulation. J Comput Chem 38:1114–1124
Jorgensen JH, Turnidge JD (2015) Susceptibility test methods: dilution and disk diffusion methods. Am Soc Microbiol 1:1253–1273
Kalhapure RS, Akamanchi KG (2012) Oleic acid based heterolipid synthesis, characterization and application in self-microemulsifying drug delivery system. Int J Pharm 425:9–18
Kalhapure RS, Sikwal DR, Rambharose S, Mocktar C, Singh S, Bester L, Oh JK, Renukuntla J, Govender T (2017) Enhancing targeted antibiotic therapy via pH responsive solid lipid nanoparticles from an acid cleavable lipid, nanomedicine nanotechnology. Biol Med 13:2067–2077
Kamaruzzaman NF, Kendall S, Good L (2017) Targeting the hard to reach: challenges and novel strategies in the treatment of intracellular bacterial infections. Br J Pharmacol 174:2225–2236
Khan HA, Ahmad A, Mehboob R (2015) Nosocomial infections and their control strategies. Asian Pac J Trop Biomed 5:509–514
Lamichhane N, Udayakumar TS, D’Souza WD, Simone CB II, Raghavan SR, Polf J, Mahmood J (2018) Liposomes: clinical applications and potential for image-guided drug delivery. Molecules 23:288
Lee H, Lim SI, Shin S-H, Lim Y, Koh JW, Yang S (2019) Conjugation of cell-penetrating peptides to antimicrobial peptides enhances antibacterial activity. ACS Omega 4:15694–15701
Leon-Sicairos N, Reyes-Cortes R, Guadrón-Llanos AM, Madueña-Molina J, Leon-Sicairos C, Canizalez-Román A (2015) Strategies of intracellular pathogens for obtaining iron from the environment. Biomed Res Int 5:15
Levison ME, Levison JH (2009) Pharmacokinetics and pharmacodynamics of antibacterial agents. Infect Dis Clin 23:791–815
Li X-Z, Nikaido H (2009) Efflux-mediated drug resistance in bacteria: an update. Drugs 69:1555–1623
Li J, Koh J-J, Liu S, Lakshminarayanan R, Verma CS, Beuerman RW (2017) Membrane active antimicrobial peptides: translating mechanistic insights to design. Front Neurosci 11:73
Li J, Xie S, Ahmed S, Wang F, Gu Y, Zhang C, Chai X, Wu Y, Cai J, Cheng G (2017) Antimicrobial activity and resistance: influencing factors. Front Pharmacol 8:364
Lin Y, Mao C (2011) Bio-inspired supramolecular self-assembly towards soft nanomaterials. Front Mater Sci 5:247
Liu X, Huang G (2013) Formation strategies, mechanism of intracellular delivery and potential clinical applications of pH-sensitive liposomes. Asian J Pharm Sci 8:319–328
Liu X, Li Z, Wang X, Chen Y, Wu F, Men K, Xu T, Luo Y, Yang L (2016) Novel antimicrobial peptide–modified azithromycin-loaded liposomes against methicillin-resistant Staphylococcus aureus. Int J Nanomed 11:6781
Lombardo D, Calandra P, Barreca D, Magazù S, Kiselev MA (2016) Soft interaction in liposome nanocarriers for therapeutic drug delivery. Nanomaterials 6:125
Malanovic N, Lohner K (2016) Antimicrobial peptides targeting gram-positive bacteria. Pharmaceuticals 9:59
Marrink SJ, Risselada HJ, Yefimov S, Tieleman DP, de Vries AH (2007) The MARTINI force field: coarse grained model for biomolecular simulations. J Phys Chem B 111:7812–7824
Martens E, Demain AL (2017) The antibiotic resistance crisis, with a focus on the United States. J Antibiot (Tokyo) 70:520
Maya S, Indulekha S, Sukhithasri V, Smitha KT, Nair SV, Jayakumar R, Biswas R (2012) International journal of biological macromolecules efficacy of tetracycline encapsulated O -carboxymethyl chitosan nanoparticles against intracellular infections of Staphylococcus aureus. Int J Biol Macromol 51:392–399
Michael CA, Dominey-Howes D, Labbate M (2014) The antimicrobial resistance crisis: causes, consequences, and management. Front Public Heal 2:145
Mishra B, Wang G (2012) Ab initio design of potent anti-MRSA peptides based on database filtering technology. J Am Chem Soc 134:12426–12429
Mishra B, Reiling S, Zarena D, Wang G (2017) Host defense antimicrobial peptides as antibiotics: design and application strategies. Curr Opin Chem Biol 38:87–96
Mishra AK, Choi J, Moon E, Baek K-H (2018) Tryptophan-rich and proline-rich antimicrobial peptides. Molecules 23:815
Mohamed MF, Abdelkhalek A, Seleem MN (2016) Evaluation of short synthetic antimicrobial peptides for treatment of drug-resistant and intracellular Staphylococcus aureus. Sci Rep 6:29707
Moiset G, Cirac AD, Stuart MCA, Marrink S-J, Sengupta D, Poolman B (2013) Dual action of BPC194: a membrane active peptide killing bacterial cells. PLoS ONE 8:e61541
Nasompag S, Dechsiri P, Hongsing N, Phonimdaeng P, Daduang S, Klaynongsruang S, Camesano TA, Patramanon R (1848) Effect of acyl chain length on therapeutic activity and mode of action of the CX-KYR-NH2 antimicrobial lipopeptide. Biochim Biophys Acta Biomembr 2015:2351–2364
Nicolas P (2009) Multifunctional host defense peptides: intracellular-targeting antimicrobial peptides. FEBS J 276:6483–6496
Omolo CA, Kalhapure RS, Jadhav M, Rambharose S, Mocktar C, Ndesendo VMK, Govender T (2017) Pegylated oleic acid: A promising amphiphilic polymer for nano-antibiotic delivery. Eur J Pharm Biopharm 112:96–108
Omolo CA, Megrab NA, Kalhapure RS, Agrawal N, Jadhav M, Mocktar C, Rambharose S, Maduray K, Nkambule B, Govender T (2019) Liposomes with pH responsive ‘on and off’ switches for targeted and intracellular delivery of antibiotics. J Liposome Res 10:1–19
Paliwal SR, Paliwal R, Vyas SP (2015) A review of mechanistic insight and application of pH-sensitive liposomes in drug delivery. Drug Deliv 22:231–242
Palm C, Netzereab S, Hällbrink M (2006) Quantitatively determined uptake of cell-penetrating peptides in non-mammalian cells with an evaluation of degradation and antimicrobial effects. Peptides 27:1710–1716
Parrinello M, Rahman A (1981) Polymorphic transitions in single crystals: a new molecular dynamics method. J Appl Phys 52:7182–7190
Qi Y, Cheng X, Han W, Jo S, Schulten K, Im W (2014) CHARMM-GUI PACE CG Builder for solution, micelle, and bilayer coarse-grained simulations. J Chem Inf Model 54:1003–1009
Rajchakit U, Sarojini V (2017) Recent developments in antimicrobial peptide conjugated gold nanoparticles. Bioconj Chem 28(11):2673–2686
Reddy PS, John MS, Devi PV, Reddy BSP (2017) Detection of vancomycin susceptibility among clinical isolates of MRSA by using minimum inhibitory concentration method. Int J Res Med Sci 3:1378–1382
Roca I, Akova M, Baquero F, Carlet J, Cavaleri M, Coenen S, Cohen J, Findlay D, Gyssens I, Heure OE (2015) The global threat of antimicrobial resistance: science for intervention. New Microbes New Infect 6:22–29
Rollin G, Tan X, Tros F, Dupuis M, Nassif X, Charbit A, Coureuil M (2017) Intracellular survival of Staphylococcus aureus in endothelial cells: a matter of growth or persistence. Front Microbiol 8:1354
Sani M-A, Separovic F (2016) How membrane-active peptides get into lipid membranes. Acc Chem Res 49:1130–1138
Schlusselhuber M, Torelli R, Martini C, Leippe M, Cattoir V, Leclercq R, Laugier C, Grötzinger J, Sanguinetti M, Cauchard J (2013) The equine antimicrobial peptide eCATH1 is effective against the facultative intracellular pathogen Rhodococcus equi in mice. Antimicrob. Agents Chemother. 1:02044
Scocchi M, Mardirossian M, Runti G, Benincasa M (2015) Non-membrane permeabilizing modes of action of antimicrobial peptides on bacteria. Curr Top Med Chem 16:76–88
Shen Y, Maupetit J (2012) PEP-FOLD : an updated de novo structure prediction server for both linear and disulfide bonded cyclic peptides. Nucleic Acids Res 40:288–293
Stubbings W, Leow P, Yong GC, Goh F, Körber-Irrgang B, Kresken M, Endermann R, Labischinski H (2011) Spectrum of activity of finafloxacin, a novel, pH-Activated Fluoroquinolone, under standard and acidic conditions. Antimicrob Agents Chemother 55:4394–4397
Tayo LL (2017) Stimuli-responsive nanocarriers for intracellular delivery. Biophys Rev 9:931–940
Tesei G, Vazdar M, Jensen MR, Cragnell C, Mason PE, Heyda J, Skepö M, Jungwirth P, Lund M (2017) Self-association of a highly charged arginine-rich cell-penetrating peptide. Proc Natl Acad Sci U S A 114:11428–11433
Thevenet P, Shen Y, Maupetit J, Guyon F, Derreumaux P, Tuffery P (2012) PEP-FOLD: an updated de novo structure prediction server for both linear and disulfide bonded cyclic peptides. Nucleic Acids Res 40:W288–W293
Turon P, Valle LJ, Alemán C, Puiggalí J (2017) Biodegradable and biocompatible systems based on hydroxyapatite nanoparticles. Appl Sci 7(1):60
van Heijenoort J (2007) Lipid intermediates in the biosynthesis of bacterial peptidoglycan. Microbiol Mol Biol Rev 71:620–635
Ventola CL (2015) The antibiotic resistance crisis: part 1: causes and threats. Pharm Ther 40:277
Wang Y, Zhao T, Wei D, Strandberg E, Ulrich AS, Ulmschneider JP (1838) How reliable are molecular dynamics simulations of membrane active antimicrobial peptides? Biochim Biophys Acta - Biomembr 2014:2280–2288
Wang S, Zeng X, Yang Q, Qiao S (2016) Antimicrobial peptides as potential alternatives to antibiotics in food animal industry. Int J Mol Sci 17:603
Ward BP, Ottaway NL, Perez-Tilve D, Ma D, Gelfanov VM, Tschöp MH, DiMarchi RD (2013) Peptide lipidation stabilizes structure to enhance biological function. Mol Metab 2:468–479
Watkins RR, Bonomo RA (2016) Overview: global and local impact of antibiotic resistance. Infect Dis Clin 30:313–322
Wen Y, Collier JH (2015) Supramolecular peptide vaccines: tuning adaptive immunity. Curr Opin Immunol 35:73–79
WHO (2014) Antimicrobial resistance: global report on surveillance. World Health Organ 12:9
Young-Speirs M, Drouin D, Cavalcante PA, Barkema HW, Cobo ER (2018) Host defense cathelicidins in cattle: types, production, bioactive functions and potential therapeutic and diagnostic applications. Int J Antimicrob Agents 8:88
Yuenyongviwat V, Ingviya N, Pathaburee P, Tangtrakulwanich B (2017) Inhibitory effects of vancomycin and fosfomycin on methicillin-resistant Staphylococcus aureus from antibiotic-impregnated articulating cement spacers. Bone Joint Res 6:132–136
Zhang H (2017) Thin-film hydration followed by extrusion method for liposome preparation. Methods Mol Biol 1522:17–22
Zhang L, Bulaj G (2012) Converting peptides into drug leads by Lipidation. Curr Med Chem 19(11):1602–1618
Zhang Q, Tang J, Ran R, Liu Y, Zhang Z, Gao H, He Q (2016) Development of an anti-microbial peptide-mediated liposomal delivery system: a novel approach towards pH-responsive anti-microbial peptides. Drug Deliv 23:1163–1170
Zhang X, Lin C, Lu A, Lin G, Chen H, Liu Q, Yang Z, Zhang H (2017) Liposomes equipped with cell penetrating peptide BR2 enhances chemotherapeutic effects of cantharidin against hepatocellular carcinoma. Drug Deliv 24:986–998
Zhu WL, Shin SY (2009) Antimicrobial and cytolytic activities and plausible mode of bactericidal action of the cell penetrating peptide penetratin and its lys-linked two-stranded peptide. Chem Biol Drug Des 73:209–215
Acknowledgements
This work was supported by the College of Health Sciences (CHS), University of KwaZulu-Natal (UKZN), UKZN Nanotechnology Platform, and the National Research Foundation (NRF) of South Africa [grant number NRF Grant No. 87790].
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare no conflict of interest.
Human and animals rights
This article does not contain any studies with human or animal participants.
Additional information
Communicated by H. S. Sharma
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Faya, M., Hazzah, H.A., Omolo, C.A. et al. Novel formulation of antimicrobial peptides enhances antimicrobial activity against methicillin-resistant Staphylococcus aureus (MRSA). Amino Acids 52, 1439–1457 (2020). https://doi.org/10.1007/s00726-020-02903-7
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00726-020-02903-7