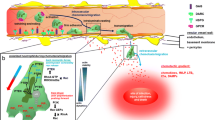
Abstract
Taurine is a free amino acid rich in neutrophils, and neutrophils play an important role in the forefront defense against infection. Upon neutrophil activation, taurine reacts with hypochlorous acid (HOCl/OCl−) produced by the myeloperoxidase (MPO) system and gets converted to taurine chloramine (Tau-Cl). Neutrophils have three types of granules, of which the primary granule MPO, secondary granule lactoferrin, and tertiary granule matrix metalloproteinase (MMP)-9 are released into the extracellular space by a process called degranulation. MPO produces hypochlorous acid to kill microorganisms, and the released MPO forms neutrophil extracellular traps (NETs) with released chromatin. Excessive secretion of MPO causes oxidative damage to the surrounding tissues. Lactoferrin exerts antioxidant activity, prevents pro-inflammatory pathway activation, sepsis, and tissue damages, and delays neutrophil apoptosis. Our experimental results show that neutrophils released small amount of granules in an inactive state, and phorbol 12-myristate 13-acetate (PMA) and N-formyl-methionine-leucyl-phenylalanine induced neutrophil degranulation. Tau-Cl inhibited the PMA-induced degranulation of MPO and formation of NETs. While Tau-Cl increased the degranulation of lactoferrin, it had no effect on MMP-9 degranulation. MPO negatively regulated the production of macrophage inflammatory protein (MIP)-2, which stimulates the degranulation and migration of neutrophils. Tau-Cl abrogated MIP-2 expression, suggestive of its inhibitory effect on MPO release. The increase in the intracellular level of MPO may negatively regulates MIP-2 expression, thereby contributing to the further regulation of neutrophil degranulation and migration. Here, we suggest that Tau-Cl selectively inhibits MPO degranulation and stimulates lactoferrin degranulation from neutrophils, thereby protecting inflamed tissues from oxidative damage induced by excessively released MPO.





Similar content being viewed by others
Data availability
The datasets generated during the current study are available from the corresponding author on reasonable request.
References
Actor JK, Hwang SA, Olsen M, Zimecki M, Hunter RL Jr, Kruzel ML (2002) Lactoferrin immunomodulation of DTH response in mice. Int Immunopharmacol 2(4):475–486
Alexander DB, Iigo M, Yamauchi K, Suzui M, Tsuda H (2012) Lactoferrin: an alternative view of its role in human biological fluids. Biochem Cell Biol 90(3):279–306
Bai L, Qiao M, Zheng R, Deng C, Mei S, Chen W (2016) Phylogenomic analysis of transferrin family from animals and plants. Comp Biochem Physiol Part D Genom Proteom 17:1–8
Baveye S, Elass E, Mazurier J, Spik G, Legrand D (1999) Lactoferrin: a multifunctional glycoprotein involved in the modulation of the inflammatory process. Clin Chem Lab Med 37(3):281–286
Bentley JK, Reed PW (1981) Activation of superoxide production and differential exocytosis in polymorphonuclear leukocytes by cytochalasins A, B, C, D and E: effects of various ions. Biochim Biophys Acta 678(2):238–244
Bentwood BJ, Henson PM (1980) The sequential release of granule constituents from human neutrophils. J Immunol 124(2):855–862
Berger G, Brekken R, McMahon G, Vu TH, Itoh T, Tamaki K, Tanzawa K, Thorpe P, Itohara S, Werb Z, Hanahan D (2000) Matrix metalloproteinase-9 triggers the angiogenic switch during carcinogenesis. Nat Cell Biol 2(10):737–744
Berlutti F, Pantanella F, Natalizi T, Frioni A, Paesano R, Polimeni A, Valenti P (2011) Antiviral properties of lactoferrin—a natural immunity molecule. Molecules 16(8):6992–7018
Borregaard N, Cowland JB (1997) Granules of the human neutrophilic polymorphonuclear leukocyte. Blood 89(10):3503–3521
Brinkmann V, Reichard U, Goosmann C, Fauler B, Uhlemann Y, Weiss DS, Weinrauch Y, Zychlinsky A (2004) Neutrophil extracellular traps kill bacteria. Science 303(5663):1532–1535
Britigan BE, Serody JS, Hayek MB, Charniga LM, Cohen MS (1991) Uptake of lactoferrin by mononuclear phagocytes inhibits their ability to form hydroxyl radical and protects them from membrane autoperoxidation. J Immunol 147(12):4271–4277
Chen Y, Hashiguchi N, Yip L, Junger WG (2006) Hypertonic saline enhances neutrophil elastase release through activation of P2 and A3 receptors. Am J Physiol Cell Physiol 290(4):C1051–1059
Dhiman M, Estrada-Franco JG, Pando JM, Ramirez-Aguilar FJ, Spratt H, Vazquez-Corzo S, Perez-Molina G, Gallegos-Sandoval R, Moreno R, Garg NJ (2009) Increased myeloperoxidase activity and protein nitration are indicators of inflammation in patients with Chagas' disease. Clin Vaccine Immunol 16(5):660–666
Gellhaar S, Sunnemark D, Eriksson H, Olson L, Galter D (2017) Myeloperoxidase-immunoreactive cells are significantly increased in brain areas affected by neurodegeneration in Parkinson’s and Alzheimer’s disease. Cell Tissue Res 369:445–454
Green PS, Mendez AJ, Jacob JS, Crowley JR, Growdon W, Hyman BT, Heinecke JW (2004) Neuronal expression of myeloperoxidase is increased in Alzheimer’s disease. J Neurochem 90(3):724–733
Heinecke JW, Li W, Francis GA, Goldstein JA (1993) Tyrosyl radical generated by myeloperoxidase catalyzes the oxidative cross-linking of proteins. J Clin Invest 91(6):2866–2872
Hickey MJ, Kubes P (2009) Intravascular immunity: the host-pathogen encounter in blood vessels. Nat Rev Immunol 9(5):364–375
Hwang SA, Actor JK (2009) Lactoferrin modulation of BCG-infected dendritic cell functions. Int Immunol 21(10):1185–1197
Kettle AJ, Winterbourn CC (1997) Myeloperoxidase: a key regulator of neutrophil oxidant production. Redox Rep 3(1):3–15
Kim C, Cha YN (2014) Taurine chloramine produced from taurine under inflammation provides anti-inflammatory and cytoprotective effects. Amino Acids 46(1):89–100
Kim C, Dinauer MC (2001) Rac2 is an essential regulator of neutrophil nicotinamide adenine dinucleotide phosphate oxidase activation in response to specific signaling pathways. J Immunol 166(2):1223–1232
Kim JW, Kim C (2005) Inhibition of LPS-induced NO production by taurine chloramine in macrophages is mediated through Ras-ERK-NF-kappa B. Biochem Pharmacol 70(9):1352–1360
Kim W, Kim HU, Lee HN, Kim SH, Kim C, Cha YN, Joe Y, Chung HT, Jang J, Kim K, Suh YG, Jin HO, Lee JK, Surh YJ (2015) Taurine chloramine stimulates efferocytosis through upregulation of Nrf2-mediated heme oxygenase-1 expression in murine macrophages: possible involvement of carbon monoxide. Antioxid Redox Signal 23(2):163–177
Kim SH, Zhong X, Kim W, Kim K, Suh YG, Kim C, Joe Y, Chung HT, Cha YN, Surh YJ (2018) Taurine chloramine potentiates phagocytic activity of peritoneal macrophages through up-regulation of dectin-1 mediated by heme oxygenase-1-derived carbon monoxide. FASEB J 32(4):2246–2257
Kleiner DE, Stetler-Stevenson WG (1994) Quantitative zymography: detection of picogram quantities of gelatinases. Anal Biochem 218(2):325–329
Kobayashi Y (2006) Neutrophil infiltration and chemokines. Crit Rev Immunol 26(4):307–316
Kobayashi SD, Voyich JM, Burlak C, DeLeo FR (2005) Neutrophils in the innate immune response. Arch Immunol Ther Exp 53(6):505–517
Korchak HM, Vienne K, Rutherford LE, Wilkenfeld C, Finkelstein MC, Weissmann G (1984) Stimulus response coupling in the human neutrophil: II. Temporal analysis of changes in cytosolic calcium and calcium efflux. J Biol Chem 259(7):4076–4082
Lacy P (2006) Mechanisms of degranulation in neutrophils. Allergy Asthma Clin Immunol 2(3):98–108
Legrand D, Elass E, Carpentier M, Mazurier J (2005) Lactoferrin: a modulator of immune and inflammatory responses. Cell Mol Life Sci 62(22):2549–2559
Löffek S, Schilling O, Franzke CW (2011) Series "matrix metalloproteinases in lung health and disease": biological role of matrix metalloproteinases: a critical balance. Eur Respir J 38(1):191–208
Marcinkiewicz J, Grabowska A, Bereta J, Stelmaszynska T (1995) Taurine chloramine, a product of activated neutrophils, inhibits in vitro the generation of nitric oxide and other macrophage inflammatory mediators. J Leukoc Biol 58(6):667–674
Oren A, Taylor JM (1995) The subcellular localization of defensins and myeloperoxidase in human neutrophils: immunocytochemical evidence for azurophil granule heterogeneity. J Lab Clin Med 125(3):340–347
Papaynnopoulos V (2018) Neutrophil extracellular traps immunity and disease. Nat Rev Immunol 18(2):134–147
Park E, Schuller-Levis G, Quinn MR (1995) Taurine chloramine inhibits production of nitric oxide and TNF-α in activated RAW 264.7 cells by mechanisms that involve transcriptional and translational events. J Immunol 154(9):4778–4784
Sato T, Hongu T, Sakamoto M, Funakoshi Y, Kanaho Y (2013) Molecular mechanisms of N-formyl-methionyl-leucyl-phenylalanine-induced superoxide generation and degranulation in mouse neutrophils: phospholipase D is dispensible. Mol Cell Biol 33(1):136–145
Schuller-Levis GB, Park E (2003) Taurine: new implications for an old amino acid. FEMS Microbiol Lett 226(2):195–202
Segal AW (2005) How neutrophils kill microbes. Annu Rev Immunol 23:197–223
Sheshachalam A, Srivastava N, Mitchell T, Lacy P, Eitzen G (2014) Granule protein processing and regulated secretion in neutrophils. Front Immunol 5:448. https://doi.org/10.3389/fimmu.2014.00448
Shimazaki KI, Kawai K (2017) Advances in lactoferrin research concerning bovine mastitis. Biochem Cell Biol 95(1):69–75
Skubitz KM (1999) Neutrophilic leukocytes. In: Lee GR, et al. (eds) Wintrobe's clinical hematology. Williams & Wilkins, Baltimore, pp 300–350
Smolen JE (1989) Characteristics and mechanisms of secretion by neutrophils. In: Hallett MB (ed) The neutrophil: cellular biochemistry and physiology. CRC Press, Boca Raton, pp 23–61
Sørensen OE, Borregaard N (2016) Neutrophil extracellular traps-the dark side of neutrophils. J Clin Invest 126(5):1612–1620
Suzuki K, Ota H, Sasagawa S, Sakatani T, Fujikura T (1983) Assay method for myeloperoxidase in human polymorphonuclear leukocytes. Anal Biochem 132(2):345–352
Takeuchi K, Umeki Y, Matsumoto N, Yamamoto K, Yoshida M, Suzuki K, Aratani Y (2012) Severe neutrophil-mediated lung inflammation in myeloperoxidase-deficient mice exposed to zymosan. Inflamm Res 61(3):197–205
Tateno N, Matsumoto N, Motowaki T, Suzuki K, Aratani Y (2013) Myeloperoxidase deficiency induces MIP-2 production via ERK activation in zymosan-stimulated mouse neutrophils. Free Radic Res 47(5):376–385
Valenti P, Antonini G (2005) Lactoferrin: an important host defense against microbial and viral attack. Cell Mol Life Sci 62(22):2576–2587
Vu TH, Shipley JM, Bergers G, Berger JE, Helms JA, Hanahan D, Shapiro SD, Senior RM, Werb Z (1998) MMP-9/gelatinase B is a key regulator of growth plate angiogenesis and apoptosis hypertrophic chondrocytes. Cell 93(3):411–422
Weiss SJ (1989) Tissue destruction by neutrophils. N Eng J Med 320(6):365–376
Wolpe SD, Sherry B, Juers D, Davatelis G, Yurt RW, Cerami A (1989) Identification and characterization of macrophage inflammatory protein 2. Proc Natl Acad Sci USA 86(2):612–616
Wong SH, Francis N, Chahal H, Raza K, Salmon M, Scheel-Toellner D, Lord JM (2009) Lactoferrin is a survival factor for neutrophils in rheumatoid synovial fluid. Rheumatol 48(1):39–44
Zawrotniak M, Rapala-Kozik M (2013) Neutrophil extracellular traps (NETs)-formation and implication. Acta Biochim Pol 60(3):277–284
Zhang R, Brennan ML, Fu X, Aviles RJ, Pearce GL, Penn MS, Topol EJ, Sprecher DL, Hazen SL (2001) Association between myeloperoxidase levels and risk of coronary artery disease. JAMA 286(17):2136–2142
Zimecki M, Właszczyk A, Zagulski T, Kübler A (1998) Lactoferrin lowers serum interleukin 6 and tumor necrosis factor alpha levels in mice subjected to surgery. Arch Immunol Ther Exp 46(2):97–104
Acknowledgements
This work was supported by the Inha University Research Grant. We thank Dr. Young June Kim (Indiana University, Indianapolis) for helpful discussion and reviewing manuscript.
Funding
This work was supported by the Inha University Research Grant.
Author information
Authors and Affiliations
Contributions
CK conceived and designed the experiments, interpreted the experimental results, and prepared the manuscript; DGK and YMK performed the experiments; and DGK, YMK, and ISK analyzed the data. All authors take responsibility for the integrity of the data analysis.
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Ethics approval
The Animal Care and Use Committee of Inha University approved this study (INHA 161214-465).
Additional information
Handling editor: S. W. Schaffer.
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Kim, D.G., Kwon, Y.M., Kang, I.S. et al. Taurine chloramine selectively regulates neutrophil degranulation through the inhibition of myeloperoxidase and upregulation of lactoferrin. Amino Acids 52, 1191–1199 (2020). https://doi.org/10.1007/s00726-020-02886-5
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00726-020-02886-5