Abstract
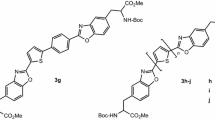
Novel thienyl and bithienyl amino acids with different substituents were obtained by a multicomponent Ugi reaction between a heterocyclic aldehyde, an amine, an acid and an isocyanide. Due to the presence of the sulphur heterocycle at the side chain, these unnatural amino acids are highly emissive and bear extra electron donating atoms so they were tested for their ability to act as fluorescent probes and chemosensors in the recognition of biomedically relevant ions in acetonitrile and acetonitrile/water solutions. The results obtained from spectrophotometric/spectrofluorimetric titrations in the presence of organic and inorganic anions, and alkaline; alkaline-earth and transition metal cations indicated that the bithienyl amino acid bearing a methoxy group is a selective colorimetric chemosensor for Cu2+, while the other (bi)thienyl amino acids act as fluorimetric chemosensors with high sensitivity towards Fe3+ and Cu2+ in a metal–ligand complex with 1:2 stoichiometry. The photophysical and ion sensing properties of these amino acids confirm their potential as fluorescent probes suitable for incorporation into peptidic frameworks with chemosensory ability.





Similar content being viewed by others
References
Batista RMF, Ferreira RCM, Raposo MMM, Costa SPG (2012) Novel optical chemosensors for anions and cations based on an amino acid core functionalised with benzimidazoles. Tetrahedron 68:7322–7330
Capobianco ML, Barbarella G, Manetto A (2012) Oligothiophenes as fluorescent markers for biological applications. Molecules 17:910–933
Castro VIB, Carvalho CM, Fernandes RDV, Pereira-Lima SMMA, Castanheira EMS, Costa SPG (2016) Peptaibolin analogues by incorporation of α, α-dialkylglycines: synthesis and study of their membrane permeating ability. Tetrahedron 72:1024–1030
Cheruku P, Huang J-H, Yen H-J, Iyer RS, Rector KD, Martinez JS, Wang H-L (2015) Tyrosine-derived stimuli responsive, fluorescent amino acids. Chem Sci 6:1150–1158
Costa SPG, Maia HLS, Pereira-Lima SMMA (2003) An improved approach for the synthesis of α, α-dialkyl glycine derivatives by the Ugi-Passerini reaction. Org Biomol Chem 1:1475–1479
Costa SPG, Oliveira E, Lodeiro C, Raposo MMM (2007) Synthesis, characterization and metal ion detection of novel fluoroiono-phores based on heterocyclic substituted alanines. Sensors 7:2096–2114
Costa SPG, Oliveira E, Lodeiro C, Raposo MMM (2008a) Heteroaromatic alanine derivatives bearing (oligo)thiophene units: synthesis and photophysical properties. Tetrahedron Lett 49:5258–5261
Costa SPG, Batista RMF, Raposo MMM (2008b) Synthesis and photophysical characterization of new fluorescent bis-amino acids bearing a heterocyclic bridge containing benzoxazole and thiophene. Tetrahedron 64:9733–9737
Dömling A (2006) Recent developments in isocyanide based multicomponent reactions in applied chemistry. Chem Rev 106:17–89
Esteves CIC, Silva AMF, Raposo MMM, Costa SPG (2009) Unnatural benz-X-azolyl asparagine derivatives as novel fluorescent amino acids: synthesis and photophysical characterization. Tetrahedron 65:9373–9377
Esteves CIC, Raposo MMM, Costa SPG (2010) Synthesis and evaluation of benzothiazolyl and benzimidazolyl asparagines as amino acid based selective fluorimetric chemosensors for Cu2+. Tetrahedron 66:7479–7486
Esteves CIC, Raposo MMM, Costa SPG (2011) Novel highly emissive non-proteinogenic amino acids: synthesis of 1,3,4-thiadiazolyl asparagines and evaluation as fluorimetric chemosensors for biologically relevant transition metal cations. Amino Acids 40:1065–1075
Esteves CIC, Batista RMF, Costa SPG, Raposo MMM (2016) Novel functionalised imidazo-benzocrown ethers bearing a thiophene spacer as fluorimetric chemosensors for metal ion detection. Dyes Pigments 135:134–142
Hennig A, Florea M, Roth D, Enderle T, Nau WM (2007) Design of peptide substrates for nanosecond time-resolved fluorescence assays of proteases: 2,3-diazabicyclo[2.2.2]oct-2-ene as a noninvasive fluorophore. Anal Biochem 360:255–265
Holler MG, Campo LF, Brandelli A, Stefani V (2002) Synthesis and spectroscopic characterisation of 2-(2’-hydroxyphenyl)benzazole isothiocyanates as new fluorescent probes for proteins. J Photochem Photobiol A Chem 149:217–225
Kajihara D, Abe R, Iijima I, Komiyama C, Sissido M, Hohsaka T (2006) FRET analysis of protein conformational change through position-specific incorporation of fluorescent amino acids. Nat Methods 3:923–929
Katritzki AR, Narindoshvili T (2009) Fluorescent amino acids: advances in protein-extrinsic fluorophores. Org Biomol Chem 7:624–627
Lee S, Xie J, Chen X (2010) Peptide-based probes for targeted molecular imaging. Biochemistry 49:1364–1376
Liu Q, Wang J, Boyd BJ (2015) Peptide-based biosensors. Talanta 136:114–127
Moragues ME, Martínez-Máñez R, Sancenón F (2011) Chromogenic and fluorogenic chemosensors and reagents for anions. A comprehensive review of the year 2009. Chem Soc Rev 40:2593–2643
Morris JV, Mahaney MA, Huber JR (1976) Fluorescence quantum yield determinations. 9,10-diphenylanthracene as a reference standard in different solvents. J Phys Chem 80:969–974
Niu W, Guo J (2013) Expanding the chemistry of fluorescent protein biosensors through genetic incorporation of unnatural amino acids. Mol Biosyst 9:2961–2970
Oliveira E, Genovese D, Juris R, Zaccheroni N, Capelo JL, Raposo MMM, Costa SPG, Prodi L, Lodeiro C (2011) Bioinspired systems for metal-ion sensing: new emissive peptide probes based on benzo[d]oxazole derivatives and their gold and silica nanoparticles. Inorg Chem 50:8834–8849
Pina JJ, de Melo SS, Batista RMF, Costa SPG, Raposo MMM (2010) Synthesis and characterization of the ground and excited states of tripodal-like oligothienyl-imidazoles. J Phys Chem B 114:4964–4972
Pless SA, Ahern CA (2013) Unnatural amino acids as probes of ligand-receptor interactions and their conformational consequences. Annu Rev Pharmacol Toxicol 53:211–229
Santos-Figueroa LE, Moragues ME, Climent E, Agostini A, Martínez-Máñez R, Sancenón F (2013) Chromogenic and fluorogenic chemosensors and reagents for anions. A comprehensive review of the years 2010–2011. Chem Soc Rev 42:3489–3613
Shimazaki Y, Takani M, Yamauchi O (2009) Metal complexes of amino acids and amino acid side chain groups. Structure and properties. Dalton Trans 38:7854–7869
Veale EB, Gunnlaugsson T (2010) Fluorescent sensors for ions based on organic structures. Annu Rep Prog Chem B Org Chem 106:376–406
Wang A, Nairn NW, Marelli M, Grabstein K (2012) Protein engineering with non-natural amino acids. In: Kaumaya P (ed) Protein engineering. InTech, Rijeka, pp 253–290
Zheng Y, Cao X, Orbulescu J, Konka V, Andreopoulos FM, Pham SM, Leblanc RM (2003) Peptidyl fluorescent chemosensors for the detection of divalent copper. Anal Chem 75:1706–1712
Zhou L, Shao J, Li Q, van Heel AJ, de Vries MP, Broos J, Kuipers OP (2016) Incorporation of tryptophan analogues into the lantibiotic nisin. Amino Acids 48:1309–1318
Acknowledgements
Thanks are due to Fundação para a Ciência e Tecnologia (FCT-Portugal) and FEDER-COMPETE for financial support through Centro de Química [PEst-C/QUI/UI0686/2013 (F-COMP-01-0124-FEDER-037302)] and a PhD grant to C.I.C. Esteves (SFRH/BD/68360/2010). The NMR spectrometer Bruker Avance III 400 is part of the National NMR Network and was purchased with funds from FCT and FEDER.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Research involving human participants and/or animals
This article does not contain any studies with human participants or animals performed by any of the authors.
Additional information
Handling Editor: T. Langer.
Electronic supplementary material
Below is the link to the electronic supplementary material.
726_2017_2392_MOESM1_ESM.docx
Supplementary data associated with this article (spectrofluorimetric titrations of compounds 2b and 2h with all the tested ions as representative examples of the behaviour of thienyl and bithienyl amino acids, respectively, and of compounds 2a-f,h-j with Fe3+; Job’s plot of compound 2i with Cu2+) can be found in the online version (DOCX 4280 kb)
Rights and permissions
About this article
Cite this article
Esteves, C.I.C., Raposo, M.M.M. & Costa, S.P.G. Non-canonical amino acids bearing thiophene and bithiophene: synthesis by an Ugi multicomponent reaction and studies on ion recognition ability. Amino Acids 49, 921–930 (2017). https://doi.org/10.1007/s00726-017-2392-7
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00726-017-2392-7