Abstract
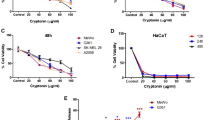
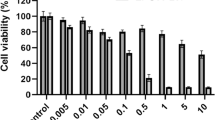
Nowadays, the emergence of resistance to the current available chemotherapeutic drugs by cancer cells makes the development of new agents imperative. The skin secretion of amphibians is a natural rich source of antimicrobial peptides (AMP), and researchers have shown that some of these wide spectrum molecules are also toxic to cancer cells. The aim of this study was to verify a putative anticancer activity of the AMP pentadactylin isolated for the first time from the skin secretion of the frog Leptodactylus labyrinthicus and also to study its cytotoxic mechanism to the murine melanoma cell line B16F10. The results have shown that pentadactylin reduces the cell viability of B16F10 cells in a dose-dependent manner. It was also cytotoxic to normal human fibroblast cells; nevertheless, pentadactylin was more potent in the first case. The studies of action mechanism revealed that pentadactylin causes cell morphology alterations (e.g., round shape and shrinkage morphology), membrane disruption, DNA fragmentation, cell cycle arrest at the S phase, and alteration of mitochondrial membrane potential, suggesting that B16F10 cells die by apoptosis. The exact mechanism that causes reduction of cell viability and cytotoxicity after treatment with pentadactylin is still unknown. In conclusion, as cancer cells become resilient to death, it is worthwhile the discovery of new drugs such as pentadactylin that induces apoptosis.








Similar content being viewed by others
References
Altschul SF, Maden TL, Schaffer AA, Zhang J, Zhang Z, Miller W, Lipman DJ (1997) Gapped BLAST and PSI-BLAST: a new generation of protein database search programs. Nucleic Acids Res 25:3389–3402
Arellano M, Moreno S (1997) Regulation of CDK/cyclin complexes during the cell cycle. Int J Biochem Cell Biol 29:559–573
Atherton E, Sheppard RC (1989) Solid phase peptide synthesis: a practical approach (the practical approach series). Oxford University Press, USA
Bhutia SK, Maiti TK (2008) Targeting tumors with peptides from natural sources. Trends Biotechnol 26:210–217
Boleti APA, Ventura CA, Justo GZ, Silva RA, Sousa ACT, Ferreira CV, Yano T, Macedo MLR (2008) Pouterin, a novel potential cytotoxic lectin-like protein with apoptosis-inducing activity in tumorigenic mammalian cells. Toxicon 51:1321–1330
Casallanovo F, De Oliveira FJ, De Souza FC, Ros U, Martínez Y, Pentón D, Tejuca M, Martínez D, Pazos F, Pertinhez TA, Spisni A, Cilli EM, Lanio ME, Alvarez C, Schreier S (2006) Model peptides mimic the structure and function of the N-terminus of the pore-forming toxin sticholysin II. Biopolymers 84:169–180
Conlon JM, Al-Dhaheri A, Al-Mutawa E, Al-Kharrge ER, Ahmed E, Kolodziejek J, Nowotny N, Nielsen PF, Davidson C (2007) Peptide defenses of the Cascades frog Rana cascadae: implications for the evolutionary history of frogs of the Amerana species group. Peptides 28:1268–1274
Cruz-Chamorro L, Puertollano MA, Puertollano E, Alvarez de Cienfuegos G, De Pablo MA (2006) In vitro biological activities of magainin alone or in combination with nisin. Peptides 27:1201–1209
Dasika GK, Lin SC, Zhao S, Sung P, Tomkinson A, Lee EYHP (1999) DNA damage-induced cell cycle checkpoints and DNA strand break repair in development and tumorigenesis. Oncogene 18:7883–7899
Dennison SR, Whittaker M, Harris F, Phoenix DA (2006) Anticancer α-helical peptides and structure/function relationships underpinning their interactions with tumour cell membranes. Curr Protein Peptide Sci 7:487–499
Doyle J, Brinkworth CS, Wegener KL, Carver JA, Llewellyn LE, Olver IN, Bowie JH, Wabnitz PA, Tyler MJ (2003) nNOS inhibition, antimicrobial and anticancer activity of the amphibian skin peptide, citropin 1.1 and synthetic modifications—the solution structure of a modified citropin 1.1. Eur J Biochem 270:1141–1153
Enbäch J, Laakkonen P (2007) Tumour-homing peptides: tools for targeting, imaging and destruction. Biochem Soc Trans 35:780–783
Fontes W, Cunha RB, Sousa MV, Morhy L (1998) Improving the recovery of lysine in automated protein sequencing. Anal Biochem 258:67–259
Gomes A, Giri B, Koleb L, Saha A, Debnath A, Gomes A (2007) A crystalline compound (BM-ANF1) from the Indian toad (Bufo melanostictus, Schneider) skin extract, induced antiproliferation and apoptosis in leukemic and hepatoma cell line involving cell cycle proteins. Toxicon 50:835–849
Guo ZS, Thorne SH, Bartlett DL (2008) Oncolytic virotherapy: Molecular targets in tumor-selective replication and carrier cell-mediated delivery of oncolytic viruses. Biochim Biophys Acta 1785:217–231
Hedges SB, Heinicke MP (2007) Molecular phylogeny and biogeography of West Indian frogs of the genus Leptodactylus (Anura, Leptodactylidae). Mol Phylogenet Evol 44:308–314
Helmerhorst EJ, Reijnders IM, Van’t-Hof W, Veerman EC, Nieuw Amerongen AV (1999) A critical comparison of the hemolytic and fungicidal activities of cationic antimicrobial peptides. FEBS Lett 449:105–110
Hoskin DW, Ramamoorthy A (2008) Studies on anticancer activities of antimicrobial peptides. Biochim Biophys Acta 1778:357–375
Hunt D, Yates JR, Shabanowitz J, Winston S, Hauer CR (1986) Protein sequencing by tandem mass spectrometry. Proc Natl Acad Sci USA 83:6233–6237
Igney FH, Krammer PH (2002) Death and anti-death: tumour resistance to apoptosis. Nat Rev Cancer 2:277–288
Kim S, Kim SS, Bang YJ, Kim SJ, Lee BJ (2003) In vitro activities of native and designed peptide antibiotics against drug sensitive and resistant tumor cell lines. Peptides 24:945–953
King JD, Al-Ghaferi N, Abraham B, Sonnevend A, Leprince J, Nielsen PF, Conlon JM (2005) Pentadactylin: An antimicrobial peptide from the skin secretions of the South American bullfrog Leptodactylus pentadactylus. Comp Biochem Physiol 141:393–397
Lam TL, Wong GKY, Chong HC, Cheng PNM, Choi SC, Chow TL, Kwok SY, Poon RTP, Wheatley DN, Lo WH, Leung YC (2009) Recombinant human arginase inhibits proliferation of human hepatocellular carcinoma by inducing cell cycle arrest. Cancer Lett 277:91–100
Mosmann T (1983) Rapid colorimetric assay for cellular growth and survival: application to proliferation and cytotoxicity assays. J Immunol Meth 65:55–63
Okada H, Mak TW (2004) Pathways of apoptotic and non-apoptotic death in tumour cells. Nat Rev Cancer 4:592–603
Pukala TL, Bowie JH, Maselli VM, Musgrave IF, Tyler MJ (2006) Host-defense peptides from the glandular secretions of amphibians: structure and activity. Nat Prod Rep 23:368–393
Riccardi C, Nicoletti I (2006) Analysis of apoptosis by propidium iodide staining and flow cytometry. Nat Protoc 1:1458–1461
Ronot X, Benel L, Adolphe M, Mounolou JC (1986) Mitochondrial analysis in living cells: the use of rhodamine 123 and flow cytometry. Biol Cell 57:1–7
Roos WP, Kaina B (2006) DNA damage-induced cell death by apoptosis. Trends Mol Med 12:440–450
Shadidi M, Sioud M (2003) Selective targeting of cancer cells using synthetic peptides. Drug Res Updates 6:363–371
Sun LK, Yoshii Y, Hyodo A, Tsurushima H, Saito A, Harakuni T, Li YP, Nozaki M, Morine N (2002) Apoptosis induced by box jellyfish (Chiropsalmus quadrigatus) toxin in glioma and vascular endothelial cell lines. Toxicon 40:441–446
Thompson JF, Scolyer RA, Kefford RF (2005) Cutaneous melanoma. Lancet 365:687–701
Tu WC, Wu CC, Hsieh HL, Chen CY, Hsu SL (2008) Honeybee venom induces calcium-dependent but caspase-independent apoptotic cell death in human melanoma A2058 cells. Toxicon 52:318–329
Zhang Y, Rishi AK, Dawson MI, Tschang R, Farhana L, Boyanapalli M, Reichert U, ShrBooot B, Buren ECV, Fontana JA (2000) S-phase arrest and apoptosis induced in normal mammary epithelial cells by a novel retinoid. Cancer Res 60:2025–2032
Zhu H, Zhang L, Wu S, Teraishi F, Davis JJ, Jacob D, Fang B (2004) Induction of S-phase arrest and p21 overexpression by a small molecule 2[[3-(2,3-dichlorophenoxy)propyl] amino]ethanol in correlation with activation of ERK. Oncogene 23:4984–4992
Acknowledgments
This study was supported in part by CNPq (Conselho Nacional de Desenvolvimento Científico e Tecnológico), CAPES (Coordenação de Aperfeiçoamento de Pessoal de Nível Superior), FINEP (Financiadora de Estudos e Projetos), FAPDF (Fundação de Apoio à Pesquisa do Distrito Federal), FAPESP (Fundação de Amparo à Pesquisa do Estado de São Paulo), FINATEC (Fundação de Empreendimentos Científicos e Tecnológicos) and FUB/UnB.
Conflict of interest statement
The authors declare that they have no conflict of interest.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Libério, M.S., Joanitti, G.A., Azevedo, R.B. et al. Anti-proliferative and cytotoxic activity of pentadactylin isolated from Leptodactylus labyrinthicus on melanoma cells. Amino Acids 40, 51–59 (2011). https://doi.org/10.1007/s00726-009-0384-y
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00726-009-0384-y