Abstract
Rheological nuclear magnetic resonance (NMR) has been applied to study the effect of shear on the chain dynamics in a solution of a linear poly(sodium 4-styrenesulfonate). Information on the chain dynamics of poly(sodium 4-styrenesulfonate) sheared in a narrow-gap concentric double cylinder cell is inferred from the NMR transverse relaxation T2 for a concentration in the semidilute-entangled and in the concentrated regime. In the semidilute-entangled concentration enhanced relaxation is observed. In the concentrated regime signatures of a loss of entanglements are seen with increasing T2 and a decreasing fraction of the polymer showing restricted motion. The effect is enhanced when the electrostatic interaction along the polymer chain is reduced by the addition of NaCl.
Similar content being viewed by others
Explore related subjects
Find the latest articles, discoveries, and news in related topics.Avoid common mistakes on your manuscript.
1 Introduction
Rheology studies the response of material to external shear forces and usually measures global properties [1, 2]. The combination with other methods allows for deriving additional microscopic information on the material under study. Scattering experiments have been combined with shear to study ordering effects [3, 4]. NMR provides a multitude of information on a molecular level [5], therefore it has been applied to complex fluids under shear [6]. Formation of multilamellar vesicles under shear has been studied for micellar systems [7, 8]. The combination of NMR with external shear provides insight into dynamics in highly viscous polymer melts [9] or to understand the Phase transition of the oil phase in an emulsion under shear [10] and the fiber formation in silk [11]. The combination of magnetic resonance imaging with pulsed-field-gradient (PFG) NMR gives access to flow patterns [12]. This has been used to study the effect of shear banding in materials with a non-linear stress response [13]. Polymer chain dynamics under external shear forces have been studied by nuclear magnetic resonance (NMR) relaxation [14]. In particular the spin–spin relaxation or transverse relaxation time T2 is sensitive to rather slow motions as are expected for polymer chain motions while the longitudinal relaxation time T1 is more sensitive to faster motion with rates in the range of the Larmor frequency [15].
Polymer melts or solutions under shear can undergo shear-induced chain orientation, they may get into a more stretched conformation or may lose a fraction of the entanglements. Shear-induced orientation or stretching would result in reduced degrees of freedom for the chains and thus reduced chain mobility, while loss of entanglements on the other hand would provide more chain mobility. In a recent study, it has been shown that in a melt of a polymer with a molecular weight above the entanglement molecular weight the chain mobility is increased under continuous shear [16]. Subsequently, it could be shown, that this happens for larger molecular weight only and does not happen for short chains [17]. In a polymer solution, there is an additional degree of freedom, the polymer concentration. To form entanglements the molecular weight has to be above the entanglement molecular weight and the concentration has to be above the entanglement concentration.
The conformation of polyelectrolytes in solution is strongly influenced by the electrostatic interaction between the charges along the polymer chain [18, 19]. This can be screened by variation of the ionic strength in the solution. The addition of salt to the solution screens the electrostatic repulsion along the chain and thus results in higher flexibility and therefore a more coiled conformation of the polymer in the solution. The aim of the investigation presented here is to study whether in solution effects like the loss of entanglements under shear are observed and to gain some insight into the influence of polymer concentration and ionic strength. We have chosen two concentrations here, one in the semidilute-entangled range and one in the concentrated range [20].
2 Experimental
The experiments were performed on a Bruker Avance III NMR spectrometer with a 7 T wide bore magnet corresponding to a Larmor frequency of 300 MHz for protons. The spectrometer is equipped with a Bruker micro 2.5 microimaging accessory allowing measurements with gradient strengths of up to 1.5 T/m in all three orthogonal directions. A birdcage resonator with an inner diameter of 15 mm was used for all experiments.
Continuous shear is applied to the sample using an in-house developed rheo NMR system as used in [21] omitting the gearbox for the oscillatory motion. It uses a servo motor permitting excellent control of the rotation speed and avoiding vibrations as would be generated by a stepper motor. A predefined list of rotation rates of the bob is set on the motor control and a TTL pulse from the NMR spectrometer advances that list allowing for the automated acquisition of a series of experiments. A concentric double-cylinder cell with a rotating inner cylinder with the sample under study in the gap also known as a Searle cell [1] has been used. The cell consists of a 10 mm glass NMR tube with an inner diameter of 8.8 mm and a rotor (bob) made from poly(ether ether ketone) (PEEK) with a diameter of 8.2 mm resulting in a gap of 300 μm. A sketch of the experimental setup is shown in Fig. 1. The velocity profile in such a cell can be described as linear under continuous shear which can be checked by flow NMR imaging [12, 21].
Technical drawing of the narrow gap concentric cylinder (Searle cell) rheological NMR experiment. (1) Vertical wide bore 7 T super-conducting NMR Magnet. (2) NMR probe with radio frequency and three axis magnetic field gradient coils that accommodates the 10 mm Searle cell. (3) servo motor generating rotational motion transmitted through a drive shaft to the central cylinder. (4) Searle sample cell
The sodium salt of poly(sodium 4-styrenesulfonate) (PSS), a flexible polyelectrolyte with an average Mw of 1,000,000 g/mol with high charge density purchased from Sigma Aldrich as powder and dried in a vacuum oven at a pressure of 200 mbar and a temperature of 60 °C. The molecular weight of the samples was chosen quite high, so that the polymer chains tend to form entanglements over a certain critical concentration of 0.07 mol/l in an aqueous solution. Therefore, the PSS was dissolved in deuterium oxide with a purity of 99.97% which was supplied by Eurisotop. To facilitate comparison to other investigations on polyelectrolytes concentrations are quoted in molar concentrations of the repeat units. One sample in semidilute-entangled range 0.5 M or 103 g/l and one sampe in the concentrated regime 2 M or 412 g/l have been used. To adjust the ionic strength individually, sodium chloride (Sigma Aldrich) was added. The samples were stirred and subsequently degassed by applying weak vacuum of up to 150 mbar for a few minutes enhancing the removal of the air bubbles. The different samples were kept at rest in the Searle cell for at least 20 min before the start of the experiments. The same time was given between the change of the shear rates to ensure the equilibration of the polymer chain dynamics.
The transverse NMR relaxation time T2 has been measured using the Carr-Purcell-Meiboom-Gill (CPMG) method [22, 23]. This multiecho-method refocuses field inhomogeneity and the possible modulation from heteronuclear J coupling. An echo time of 500 μs has been used with a refocusing (π pulse) of 30 μs has been chosen. In the two-dimensional experiment, the number of echoes has been incremented in a logarithmic series to accommodate the expected exponential signal decay. The experiment is performed in a pseudo-two-dimensional fashion, for each number of echoes a full NMR signal is detected. For each echo train an FID has been acquired so that after Fourier transform a full spectrum is detected for each echo time allowing evaluation of the decay for individual sites identified by the chemical shift. It is important here to separate the solvent with its much longer T2 from the polymer under study.
Data processing was performed in TopSpin and in-house written Matlab scripts, including functions of the free software package matNMR. The FID signals have been exponentially apodized by up to 30 Hz (0.1 ppm) and Fourier transformed. The aromatic peak at 7.7 ppm has been integrated. Data points below a signal-to-noise ratio of 5 have been neglected.
3 Results and Discussion
For the current system, there are two factors limiting the concentration range that can be studied. At a high concentration beyond 2 M the viscoelastic nature of the polymer becomes important resulting in a severe Weissenberg effect. The Weissenberg effect scales with the rotation frequency of the bob rather than the shear rate. Therefore, after initial experiments the setup has been modified for the minimal practical gap width of 300 μm to establish the required shear rates. At higher polymer concentrations the Weissenberg effect sets in at low shear rates meaning the solution climbs on the bob and the resulting gap in the material hinders the shear.
At low concentration, both the sensitivity and the dynamic range become limiting. Sensitivity or signal-to-noise ratio could be improved by more repetitions of the experiments that finally is limited by the stability of the sample. Even though the experiments have been performed in deuterated water there is a residual proton signal that becomes significant at low concentrations. The water signal exhibits a rather long T2 so that the residual becomes more relevant at the decaying signal. The lineshape in the rheo NMR signal is hampered by the double cylinder setup and the resulting multiple material interfaces compared to a high-resolution NMR setup.
A typical series of signal decays under the CPMG sequence is depicted in Fig. 2 for the concentrated, 2 M, solution of PSS with 1 M NaCl. The experiment at rest has been repeated at the end showing the same result as at the beginning. This proves that the effects are reversible.
In polymers usually a distribution of relaxation times is observed due to the disordered nature. Shorter T2 is indicative of restricted chain motion that appears in the vicinity of entanglements while longer T2 is indicative of more free chain motion. There are various methods to analyze such a distribution of exponential decay. The data observed here are readily described by a two-component exponential decay as described in Eq. 1.
with the two relaxation times T2a andT2b and the relative population A. With the two-component, three-parameter, fit there is a residual of less than 3% of the total signal intensity that can be readily ignored for the analysis here. The relaxation NMR experiment is a quantitative experiment, all protons of the polymer contribute with equal intensity to the signal. Thus, the relative population is a measure of the fraction of the polymer experiencing restricted motion.
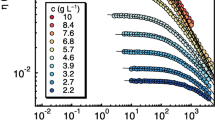
The transverse relaxation time of PSS has been measured for different concentrations of PSS in aqueous solution. With increasing polymer concentration there is increasing interaction between the polymers hindering the motion [24] and thus both T2 decay components become shorter while there is no clear trend in the relative population.
Concentrations of PSS well below the entanglement concentration [25] are currently not really accessible for the experiment reported here. Even using presaturation of the remaining water signal the dynamic range is not sufficient to detect two components in the exponential signal decay of the signal originating from the polymer. No significant change of the apparent T2 decay with shear has been detected, which could result from only a small shear effect at the low concentration or an apparent cancelation of the effects of orientation and loss of entanglements.
The fit results for 0.5 M PSS are depicted in Fig. 3. The time constants for both components become longer with shear, while the fraction A exhibiting the faster relaxation decay increases as well. As a result in both samples, the signal decay in the CPMG experiment becomes faster. In the case with the added salt the increase in all three parameters sets in at higher shear rates.
At a significantly higher concentration, 2 M, a different picture is observed as seen in Fig. 4. Thought the time constant for the faster T2a decay increases there is not such a clear trend for the slower T2b. More significantly here the fraction exhibiting the shorter signal decay is initially lower than in the salt-free sample and becomes even lower under shear.
In the case of added salt screening the electrostatic interaction along the chain thus making the chain more flexible the results follow the trend previously seen for polymer melts. A summary of the fit results is given in Table 1.
4 Conclusions
Rheological NMR has been used to gain insight into the polymer chain dynamics in aqueous solutions of poly(sodium 4-styrenesulfonate) under shear in a Searle cell. For a solution of a polyelectrolyte the concentration of the polymer has a significant impact. In addition, the electrostatic repulsion along the polymer chain and thus the stiffness of the polymer can be influenced by the ionic strength.
The transverse NMR relaxation time T2 has been used to get information on the polymer chain dynamics. T2 has been proven to be a very useful measure over a wide range of correlation times or residual dipolar couplings in the system under study.
Low concentration of PSS, well below the entanglement concentration, are not fully accessible with the current experiment. At an intermediate polyelectrolyte concentration there is a slight increase of the two components of the relaxation time, however, the fraction that exhibits the shorter relaxation time increases simultaneously leading to a faster signal decay indicative of restrictions of the motion.
At concentrations above the entanglement concentration upon shear both time constants for the relaxation time increase and perhaps more importantly the fraction of the material exhibiting the faster relaxation decreases. Increasing transverse NMR relaxation time indicates enhanced chain mobility. A reduction of the fraction of the polymer chain experiencing restricted motion is in line with a loss of entanglements as it has been observed for polymer melts. Increasing the flexibility of the polyelectrolyte chain by the addition of salt enhances the effect.
Availability of Data and Materials
Data will be made available upon reasonable request, All data are stored at IPF.
References
C.W. Macosko, Rheology principles, measurements, and applications (VCH, New York, 1994)
R. Brummer, Rheology essentials of cosmetics and food emulsions (Springer, Amsterdam, 2005)
P.E. Boukany, S.-Q. Wang, Nature of steady flow in entangled fluids revealed by superimposed small amplitude oscillatory shear. J. Rheol. 53(6), 1425–1435 (2009)
T. Meins, K. Hyun, N. Dingenouts, M. FotouhiArdakani, B. Struth, M. Wilhelm, New insight to the mechanism of the shear-induced macroscopic alignment of diblock copolymer melts by a unique and newly developed Rheo–SAXS combination. Macromolecules 45(1), 455–472 (2011)
B. Blümich, NMR imaging of materials, vol. 57 (Oxford University Press, Oxford, 2003)
P.T. Callaghan, Rheo-NMR: nuclear magnetic resonance and the rheology of complex fluids. Rep. Prog. Phys. 62, 599–670 (1999)
S. Kuczera, L. Gentile, T.I. Brox, U. Olsson, C. Schmidt, P. Galvosas, Multilamellar vesicle formation probed by Rheo-NMR and Rheo-SALS under large amplitude oscillatory shear. Langmuir 34(28), 8314–8325 (2018)
B. Medronho, J. Brown, M.G. Miguel, C. Schmidt, U. Olsson, P. Galvosas, Planar lamellae and onions: a spatially resolved rheo–NMR approach to the shear-induced structural transformations in a surfactant model system. Soft Matter 7(10), 4938 (2011)
V. Rantzsch, M. Wilhelm, G. Guthausen, Hyphenated low-field NMR techniques: combining NMR with NIR GPC/SEC and rheometry. Magn. Reson. Chem. 54(6), 494–501 (2016)
G. Kaysan, B. Spiegel, G. Guthausen, M. Kind, Influence of shear flow on the crystallization of organic melt emulsions—a rheo-nuclear magnetic resonance investigation. Chem. Eng. Technol. (2020). https://doi.org/10.1002/ceat.202000193
F.B. Kosuke Ohgo, Tetsuo Asakura, Ulrich Scheler investigation of structural transition of regenerated silk fibroin aqueous solution by Rheo-NMR spectroscopy. J Am Chem Soc 130, 4182–4186 (2008)
P.T. Callaghan, Principles of nuclear magnetic resonance microscopy (Oxford Science Publications, Oxford, 1991)
M.M. Britton, P.T. Callaghan, Shear banding instability in wormlike micellar solutions. Eur. Phys. J. B. 7, 237–249 (1999)
V.D.H.S. Fedotov, In NMR basic principles and progress (Springer, Heidelberg, 1989)
H. Luo, M. Klüppel, H. Schneider, Study of filled SBR elastomers using NMR and mechanical measurements. Macromolecules 37, 8000–8009 (2004)
U. Böhme, K. Gelfert, U.Scheler, Solid-State NMR on Polymers under Mechanical Stress. 109–112 (2011)
B. Wiesner, B. Kohn, M. Mende, U. Scheler, Polymer Chain Mobility under Shear-A Rheo-NMR Investigation. Polymers 10, 1231 (2018)
Visakh P.M. Oguz Bayraktar, G. A. P., Polyelectrolytes Thermodynamics and Rheology. Springer: Cham Heidelberg New York Dordrecht London, 2014.
U. Böhme, U. Scheler, Effective charge of poly(styrenesulfonate) and ionic strength—an electrophoresis NMR investigation. Colloids Surf. A 222(1–3), 35–40 (2003)
A. Matsumoto, C. Zhang, F. Scheffold, A.Q. Shen, Microrheological approach for probing the entanglement properties of polyelectrolyte solutions. ACS Macro Lett. 11(1), 84–90 (2021)
E.W. Benjamin Kohn, Kenji sugase, daichi morimoto, ulrich scheler, counter-flow phenomena studied by nuclear magnetic resonance (NMR) velocimetry and flow simulations. Phys. Fluids 34, 073608 (2022)
H.Y. Carr, E.M. Purcell, Effects of diffusion on free precession in nuclear magnetic resonance experiments. Phys. Rev. 94, 630 (1954)
S. Meibom, D. Gill, Modified spin-echo method for measuring nuclear relaxation times. Rev. Sci. Instrum. 29, 688 (1959)
A. Sagidullin, B. Fritzinger, U. Scheler, V.D. Skirda, Self-diffusion of low-generation PAMAM dendrimers with hydroxyl surface groups in solutions: a general regularity. Polymer 45(1), 165–170 (2004)
C.B. David, H.C. Ralph, Rheology of sulfonated polystyrene solutions. Macromolecules 31, 5746–5755 (1998)
Acknowledgements
We thank Enno Stündel for their support making the motor control as well as Vincent Körber and the IPF machine shop for the rheo NMR Searle cell.
Funding
Open Access funding enabled and organized by Projekt DEAL. Not applicable.
Author information
Authors and Affiliations
Contributions
SB and BK performed experiments and data analysis, US designed and supervised the research. All contributed to the manuscript.
Corresponding author
Ethics declarations
Conflict of Interest
None.
Ethical Approval
Not applicable.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Prepared for Applied Magnetic Resonance issue on the occasion of Bernhard Blümich’s 70th birthday.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Bartosch, S., Kohn, B. & Scheler, U. Chain Dynamics in a Polyelectrolyte Solution Under Shear: A Rheological NMR Investigation. Appl Magn Reson 54, 1533–1541 (2023). https://doi.org/10.1007/s00723-023-01598-9
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00723-023-01598-9