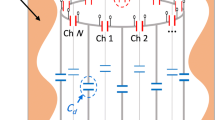
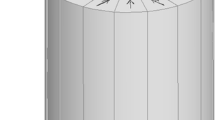
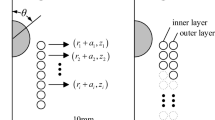
Abstract
Ultra-low magnetic field systems have attracted increasing attention in recent years because they lack cryocoolers and exhibit lightweight, low cost, and mobilization convenience. In contrast to permanent magnet systems, electromagnetic systems can easily vary background magnetic fields, thus offering opportunities on multiple scales of time and distance to characterize the molecular dynamics and transport properties of complex liquids in bulk or embedded in confined environments. An unconventional ultra-low field bell-shaped head MRI magnet with a dedicated compact structure is needed for applications in clinics, intensive care units, and mobile vans and evaluating stroke in the hyperacute and acute stages. In this work, we use the discrete minimum energy (DME) method to design the ultra-low field bell-shaped head MRI electromagnet. This method involves obtaining a DME current map, initial coil extraction, and coil adjustment. The DME map is used to determine the initial current distribution of the MRI electromagnet over a predefined domain subject to constraints. Then, initial coil extraction aims to extract the initial regularized rectangle coil layout from the DME map. Considering that the extraction process decreases magnetic field homogeneity in the region of interest (ROI), coil adjustment is then implemented. This step adjusts the position and volume of the initial coil layout to meet the homogeneity requirement. A bell-shaped electromagnet (\(< 20\) mT, 2 MA/m\(^2\)) with a homogeneity of 7 ppm over 260 mm DSV is designed in this work.







Similar content being viewed by others
References
M. Zhou, H. Wang, J. Zhu, W. Chen, L. Wang, S. Liu, Y. Li, L. Wang, Y. Liu, P. Yin, J. Liu, S. Yu, F. Tan, R.M. Barber, M.M. Coates, D. Dicker, M. Fraser, D. González-Medina, H. Hamavid, Y. Hao, G. Hu, G. Jiang, H. Kan, A.D. Lopez, M.R. Phillips, J. She, T. Vos, X. Wan, G. Xu, L.L. Yan, C. Yu, Y. Zhao, Y. Zheng, X. Zou, M. Naghavi, Y. Wang, C.J.L. Murray, G. Yang, X. Liang, Cause-specific mortality for 240 causes in China during 1990–2013: a systematic subnational analysis for the Global Burden of Disease Study 2013. The Lancet 387(10015), 251–272 (2016). https://doi.org/10.1016/S0140-6736(15)00551-6
H. Wang, Global, regional, and national life expectancy, all-cause mortality, and cause-specific mortality for 249 causes of death, 1980–2015: a systematic analysis for the Global Burden of Disease Study 2015. The Lancet 388, 86 (2016)
K.J. Becker, A.B. Baxter, H.M. Bybee, D.L. Tirschwell, T. Abouelsaad, W.A. Cohen, Extravasation of radiographic contrast is an independent predictor of death in primary intracerebral hemorrhage. Stroke 30(10), 2025–2032 (1999). https://doi.org/10.1161/01.STR.30.10.2025
C.J. van Asch, M.J. Luitse, G.J. Rinkel, I. van der Tweel, A. Algra, C.J. Klijn, Incidence, case fatality, and functional outcome of intracerebral haemorrhage over time, according to age, sex, and ethnic origin: a systematic review and meta-analysis. Lancet Neurol. 9(2), 167–176 (2010). https://doi.org/10.1016/S1474-4422(09)70340-0
S. Makkat, J.E. Vandevenne, G. Verswijvel, T. Ijsewijn, M. Grieten, Y. Palmers, A.M. De Schepper, P.M. Parizel, Signs of acute stroke seen on fluid-attenuated inversion recovery MR imaging. Am. J. Roentgenol. 179(1), 237–243 (2002). https://doi.org/10.2214/ajr.179.1.1790237
L.L. Wald, P.C. McDaniel, T. Witzel, J.P. Stockmann, C.Z. Cooley, Low-cost and portable MRI. J. Magn. Reson. Imaging 52(3), 686–696 (2020). https://doi.org/10.1002/jmri.26942
S. Geethanath, J.T. Vaughan, Accessible magnetic resonance imaging: a review. J. Magn. Reson. Imaging 49(7), e65–e77 (2019). https://doi.org/10.1002/jmri.26638
J.P. Marques, F.F. Simonis, A.G. Webb, Low-field MRI: an MR physics perspective. J. Magn. Reson. Imaging 49(6), 1528–1542 (2019). https://doi.org/10.1002/jmri.26637
T. O’Reilly, W. Teeuwisse, A. Webb, Three-dimensional MRI in a homogenous 27 cm diameter bore Halbach array magnet. J. Magn. Reson. 307, 106578 (2019). https://doi.org/10.1016/j.jmr.2019.106578
C.Z. Cooley, P.C. McDaniel, J.P. Stockmann, S.A. Srinivas, S. Cauley, M. Śliwiak, C.R. Sappo, C.F. Vaughn, B. Guerin, M.S. Rosen, M.H. Lev, L.L. Wald, A portable brain MRI scanner for underserved settings and point-of-care imaging (2019), p. 18
Y. He, W. He, L. Tan, F. Chen, F. Meng, H. Feng, Z. Xu, Use of 2.1 MHz MRI scanner for brain imaging and its preliminary results in stroke. J. Magn. Reson. 319, 106829 (2020). https://doi.org/10.1016/j.jmr.2020.106829
J.P. Korb, Multiscale nuclear magnetic relaxation dispersion of complex liquids in bulk and confinement. Prog. Nucl. Magn. Reson. Spectrosc. 104, 12–55 (2018). https://doi.org/10.1016/j.pnmrs.2017.11.001
M.J. MacLeod, P.J. Ross, G. Guzman-Guttierez, D.J. Lurie, L.M. Broche, Imaging of acute stroke by FFC-MRI: the PUFFINS study (2019), p. 3
S. Lother, S.J. Schiff, T. Neuberger, P.M. Jakob, F. Fidler, Design of a mobile, homogeneous, and efficient electromagnet with a large field of view for neonatal low-field MRI. Magn. Reson. Mater. Phys. Biol. Med. 29(4), 691–698 (2016). https://doi.org/10.1007/s10334-016-0525-8
M. Sarracanie, C.D. LaPierre, N. Salameh, D.E.J. Waddington, T. Witzel, M.S. Rosen, Low-cost high-performance MRI. Sci. Rep. 5(1), 15177 (2015). https://doi.org/10.1038/srep15177
Y.C.N. Cheng, T.P. Eagan, R.W. Brown, S.M. Shvartsman, M.R. Thompson, Design of actively shielded main magnets: an improved functional method. Magn. Reson. Mater. Phys. Biol. Med. 16(2), 57–67 (2003). https://doi.org/10.1007/s10334-003-0012-x
A. Kalafala, A design approach for actively shielded magnetic resonance imaging magnets. IEEE Trans. Magn. 26(3), 1181–1188 (1990). https://doi.org/10.1109/20.53996
S. Crozier, Compact MRI magnet design by stochastic optimization (2019), p. 5
N. Shaw, R. Ansorge, Genetic algorithms for MRI magnet design. IEEE Trans. Appl. Supercond. 12(1), 733–736 (2002). https://doi.org/10.1109/TASC.2002.1018506
M. Thompson, R. Brown, V. Srivastava, An inverse approach to the design of MRI main magnets. IEEE Trans. Magn. 30(1), 108–112 (1994). https://doi.org/10.1109/20.272522
H. Zhao, S. Crozier, D.M. Doddrell, A hybrid, inverse approach to the design of magnetic resonance imaging magnets. Med. Phys. 27(3), 599–607 (2000). https://doi.org/10.1118/1.598899
H. Zhao, S. Crozier, D.M. Doddrell, Compact clinical MRI magnet design using a multi-layer current density approach (2019), p. 10
Q. Wang, Practical Design of Magnetostatic Structure Using Numerical Simulation: Wang/Practical Design of Magnetostatic Structure Using Numerical Simulation. (Wiley, Singapore, 2013). https://doi.org/10.1002/9781118398159
H. Wheeler, Simple inductance formulas for radio coils. Proc. IRE 16(10), 1398–1400 (1928). https://doi.org/10.1109/JRPROC.1928.221309
J. Nocedal, S.J. Wright, Numerical Optimization, 2nd Ed. Springer Series in Operations Research (Springer, New York, 2006).
N. Otsu, A threshold selection method from gray-level histograms (2019), p. 5
R. Sedgewick, K.D. Wayne, Algorithms, 4th edn. (Addison-Wesley, Upper Saddle River, 2011).
P.E. Gill, W. Murray, M.A. Saunders, SNOPT: an SQP algorithm for large-scale constrained optimization. SIAM Rev. 47(1), 99–131 (2005). https://doi.org/10.1137/S0036144504446096
L. Tsai, R. Mair, M. Rosen, S. Patz, R. Walsworth, An open-access, very-low-field MRI system for posture-dependent 3He human lung imaging. J. Magn. Reson. 193(2), 274–285 (2008). https://doi.org/10.1016/j.jmr.2008.05.016
Acknowledgements
This work was supported by the National Natural Science Foundation of China (Grant no. 52077023) and the Fundamental Research Funds for the Central Universities (nos. 2018CDJDDQ0017, 2019CDYGYB001). (Corresponding author: Zheng Xu and Pan Guo.)
Author information
Authors and Affiliations
Corresponding authors
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Ding, Y., Guo, P., Wu, J. et al. Simulation and Optimization Study of an Ultra-Low-Field Bell-Shaped Head MRI Electromagnet. Appl Magn Reson 52, 691–704 (2021). https://doi.org/10.1007/s00723-021-01341-2
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00723-021-01341-2