Abstract
Experimental spectroscopic and magnetic data for Co2+(3d7) ions in various systems are reviewed and critically examined. The focus is on Co2+ ions with the electronic spin S = 3/2, properties of which may be interpreted using the spin Hamiltonian with the effective S̃ = 3/2 or the fictitious ‘spin’ S (S′) = ½. Possible distinct ground states of Co2+(3d7) ions arising from crystal field energy levels are discussed. Distinctions between the concepts of the effective spin S̃ and the fictitious ‘spin’ S′ are outlined to clarify the terminological confusion encountered in literature. Sample cases of the ground state assignments and options for the ‘spin’ S′ = ½ origin are considered for better understanding of the Co2+ ions local environment in various systems, including low symmetry cases. Present study is motivated by potential applications of Co2+(S̃ = 3/2) complexes exhibiting very large or moderate zero-field splitting as molecular nanomagnets.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
1 Introduction
The electron magnetic resonance (EMR) spectra [1, 2] of various single crystals doped with Co2+(3d7) ions with the electronic spin S = 3/2, which yields nominally the effective S̃ = 3/2, should be described by the spin Hamiltonian (SH) including the zero-field splitting (ZFS) terms [3]. However, often Co2+ EMR spectra are interpreted using SH with the ‘spin’ S (S′) = ½, which includes only the Zeeman and hyperfine structure tensors g and A. In a number of papers, the origin of the observed ground Kramers doublet state, to which the so called ‘spin’ S′ = ½ is ascribed, is not specified.
The preliminary considerations were previewed in a poster presentation at the V Forum EMR-PL [4]. Here, we elucidate key aspects and present sample data. The possible mechanisms leading to such distinct ground Co2+(3d7) states are overviewed in terms of the underlying crystal field (CF) energy level schemes. The crucial concepts of the effective spin S̃ and the fictitious ‘spin’ S′ (that in fact does not represent the true spin) are clarified. The distinction between these concepts helps in clarifying the terminological confusion encountered in literature. Specific cases of assignments of the ground states and options for the origin of the ‘spin’ S′ = ½ are considered to obtain better understanding of the local environment around the Co2+ ions in various host crystals, including low-symmetry cases.
Additional motivation for the wider study of Co2+ (3d7) ions in crystals arises from realization of potential applications of Co2+ complexes, which exhibit a variety of behavior associated with either the effective spin S̃ = 3/2 or the fictitious ‘spin’ S′ = ½. This offers potential applications ranging from the Co2+ compounds, which may be suitable as high-pressure probes for high-magnetic field and high-frequency EMR (HFM–EMR) measurements [5,6,7], to Co2+-based molecular nanomagnets exhibiting very large or moderate ZFS, including single-molecule magnets (SMM) and single-ion magnets (SIM) [8]. As a case study, we investigate: (1) the polarized optical absorption and EMR spectra of Co-doped beryls and chrysoberyl [9], (2) EMR spectra of Co2+ (S′ = ½) ions in PbMoO4 and YAlO3 [10, 11]. The calculations are carried out using the crystal field analysis (CFA) package [12], which enables the complete diagonalization within the whole 3dN configuration for arbitrary symmetry. The lowest energy levels and corresponding wave functions enable identifications of the particular observed ground Kramers doublet states. This mini review provides a primer for experimentalists to properly understand the crucial concepts of the effective spin S̃ and the fictitious ‘spin’ S′ for analysis and interpretation of EMR data. Full results and detailed considerations will be provided in subsequent publication.
2 Pertinent Aspects of Crystal Field (CF) and Spin Hamiltonian (SH) Theory
2.1 Distinction Between the High-Spin (S = 3/2) and the Low-Spin (S = ½) Configurations of Co2+(3d7) Ions
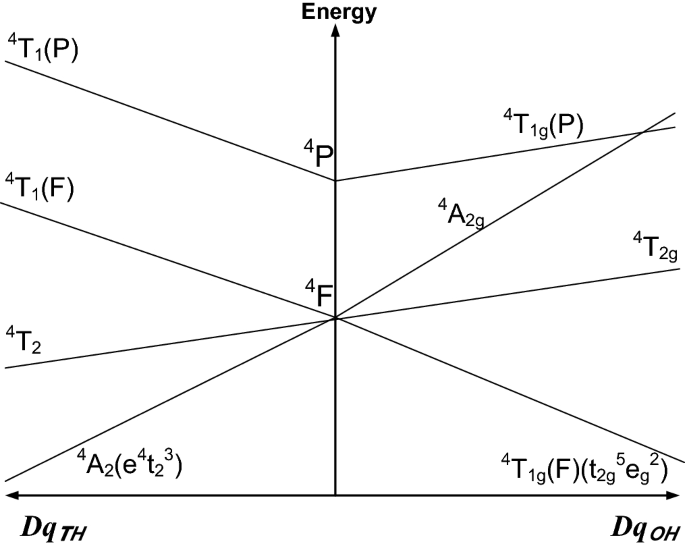
The Co2+(3d7) ions in crystals occur in one of the two ‘configurations’ or ‘states’: HS = the high-spin (S = 3/2) or LS = the low-spin (S = ½) configuration [1, 13,14,15,16,17,18,19,20]. Here, the ‘spin’ S means the (total) electronic spin. The electronic configuration describes the way in which the five d-orbitals (e, t2) of the Co2+(3d7) ion in the cubic symmetry CF [13,14,15,16,17,18,19,20,21] are occupied by electrons with the spin up (↑) and down (↓). For illustration, the two high-spin (S = 3/2) cases arising in tetrahedral (TH) and octahedral (OH) coordination [19] are presented in Fig. 1. Below, we discuss a few intricate points for the benefit of non-expert readers.
Schematic variation of the energy levels for Co2+ ion at tetrahedral (TH) and octahedral (OH) sites with an increasing cubic CF parameter Dq; adapted from [19]
For the pure octahedral (OH), i.e., sixfold, coordination in the ‘weak’ CF the configuration (t2g)5(e)2 with three unpaired electrons arises yielding the d-electron spins arranged as (↑↓, ↑↓, ↑) (↑, ↑) and the ground state is 4T1(g)(4F) with the high-spin (S = 3/2) and threefold orbital degeneracy. In the ‘strong’ OH CF, the configuration (t2)6(e)1 arises with one unpaired electron (↑↓,↑↓, ↑↓) (↑) yielding the ground term 2E(g)(2G) with the low-spin (S = ½) and twofold orbital degeneracy; see, e.g., the Table 6.1 of [22]. Note an inconsistency for the ‘strong’ OH CF case in the Table 3.1 of [23], where the spin value ‘1’ should be replaced by ‘½’ [22, 23]. As an example, the three- and one unpaired electron form the compounds [(NH4)2Co(SO4)2.6H2O] and [K2BaCo(NO2)6], respectively, are named in [22, p. 128]. The correlation diagrams, see Figs. 6.5 and 6.6 in [22], between the energy levels for various 3dN ions in the ‘weak’ and ‘strong’ CF (both for the octahedral and tetrahedral coordination discussed below) illustrate nicely also the two Co2+ configurations.
For the tetrahedral coordination (fourfold) and ‘cubic’ coordination (eightfold) [24], in the ‘weak’ CF the configuration (e)4(t2)3 with three unpaired electrons arises yielding the d-electron spins arranged as (↑↓, ↑↓) (↑, ↑, ↑), and thus the ground state is 4A2(g)(F) with the high spin (S = 3/2); as an example the compound Cs2CoCl4 is named in [22, p. 127]. For the TH coordination in the ‘strong’ CF the arrangement of the d-electron spins is controversial in view of several inconsistencies in the textbooks [13,14,15,16,17,18,19,20,21,22,23]. Nominally, by analogy, the configurations (e)4(t2)3 with one unpaired electron could be expected yielding (↑↓, ↑↓) (↑↓, ↑) and thus, the ground state would be 2E(g) with the low spin (S = ½). However, doubts arise if this configuration is feasible. It appears that in the Table 2.3 of [23] the last arrow (↑) should be omitted and the number of unpaired electrons should be one not three. In the Table 3.2 of [2], the entries for the ‘weak’ and ‘strong’ CF case are identical and correspond to the high-spin case only. In the Table 6.1 of [20], the entry for the ‘strong’ CF case reads as that listed above for the ‘weak’ CF, but in the Table 9.3 of [22], where the ‘strong’ or ‘weak’ CF is not specified, only one case is listed: [(e)4(t2)3, 4A2]. In the Table 3 of [19], no low-spin case is listed for 3d7 ion in the TH coordination. Note that the Table 2.3 of [23] refers only to the ‘tetrahedral coordination’, whereas the corresponding Table 3.2 of [2] to the ‘4-, 8- and 12-coordination’. The controversy may be solved based on the correlation diagrams between the energy levels for Co2+(3d7) ion in the ‘strong’ CF and TH coordination, see Fig. 6.5 in [22]. In this case, the configuration (e)4(t2)3 arises yielding the ground state as 4A2(g)(F), i.e., also the high-spin (S = 3/2) configuration, however, with different excited states than for the TH ‘weak’ CF case (see Fig. 1 below), since they arise from the higher term 2G as 2E(g), 2T1(g) and 2T2(g) [22]. Hence, based on the survey of textbooks and reviews, the existence of the low-spin configuration (↑↓, ↑↓) (↑↓, ↑) for Co2+(3d7) ions in the TH coordination in the ‘strong’ CF may be rather excluded.
The inversion of the CF energy levels for the sixfold coordination as compared with those for the four- and eightfold coordination is implied by the negative sign in the relationships, predicted based on the point charge model, between the octahedral cubic CF parameter, Dq(6), and that for the four- and eightfold coordination: Dq(8) = − (8/9)Dq(6) and Dq(4) = − (4/9)Dq(6), see, e.g., [13,14,15, p.468; 16, p. 62; 22, p. 41].
2.2 Distinction Between the Electronic Spin, the Effective Spin, and the Fictitious ‘Spins’
The crux of these problems appears to be the general lack of proper distinction between the various meanings of the ‘spin’, which were clearly categorized in the reviews [25, 26] and most recently in [3]. Importantly, the electronic spin S = ½ ascribed to the low-spin Co2+(3d7) configuration has to be distinguished from other ‘spins’ X, i.e., the effective spin \(\tilde{S}\) = ½ and the fictitious ‘spin’ S′ = ½, which may be ascribed to the specific subsets of Co2+ ground states with degeneracy, i.e., multiplicity (2X + 1) equal to two. As exemplified and discussed in details in the reviews [3, 25, 26], the meanings of the terms: physical versus effective Hamiltonian as well as electronic versus effective versus fictitious spin, are not well defined and are often confused with each other in the EMR-related literature.
Due to the action of the physical Hamiltonian [1,2,3], consisting of the free ion (FI) and CF terms: Hphys = HFI+ HCF, the ground state of a transition-metal ion in crystal may be either an orbital singlet (A) or an orbitally degenerate state (E, T), e.g., 4A2(F) and 4T1g(F), respectively, in Fig. 1. The orbital singlet ground state may be shortly denoted \(\left| {\left. \gamma \right\rangle } \right.\, \equiv \,\left\{ {\left| {\left. {\varGamma_{\gamma } } \right\rangle } \right.\,\left| {S, \, \left. {M_{S} \,} \right\rangle } \right.} \right\}\), where \(|\,\left. {\varGamma_{\gamma } } \right\rangle \,\) represents the orbital part, whereas \(\left| {\left. {S, \, M_{S} } \right\rangle } \right.\,\,\)—the spin part. Note that Hphys acts within the basis of the physical states of the whole configuration nlN, including the singlet \(\left| {\left. \gamma \right\rangle } \right.\). The transitions between the spin levels within the \(\left| {\left. \gamma \right\rangle } \right.\) states are observed using EMR [1,2,3, 13,14,15,16,17,18], whereas those between \(\left| {\left. \gamma \right\rangle } \right.\) and the higher lying multiplets (see, Fig. 1) using optical spectroscopy [19,20,21,22,23]. To describe only the former transitions, without being bothered with the higher lying levels, the concept of the effective spin Hamiltonian Heff has been introduced [1,2,3, 13,14,15,16,17,18, 25,26,27]. Important point is that the effective SH, unlike Hphys, acts only within its own basis of states, i.e., \(\left| {\left. {\tilde{S},\,\tilde{M}_{s} } \right\rangle } \right.\) of the effective spin operator S̃. Major advantage of Heff is that it mimics the energy levels of the electronic spin states \(\left| {\left. {S, \, M_{S} } \right\rangle } \right.\,\,\) [3, 25,26,27]. To emphasize the different nature of the physical and effective quantities, the tilde (~) is used to distinguish the effective spin operator S̃ and its states from \(\left| {\left. {\tilde{S},\,\tilde{M}_{s} } \right\rangle } \right.\) the (total) electronic spin operator S and its states \(\left| {\left. {S, \, M_{S} } \right\rangle } \right.\,\,\). Since the values of the quantum numbers S and \(\tilde{S}\) are equal for the transition ions with an orbitally non-degenerate ground state, often the two types of related quantities are inappropriately mixed up in the EMR literature. The respective reviews may be consulted for a concise description of the microscopic spin Hamiltonian (MSH) theory, which underlies the original derivation of the effective SH Heff; and the interrelationships between HCF and HZFS [3, 25,26,27] as well as the low symmetry aspects in EMR [28].
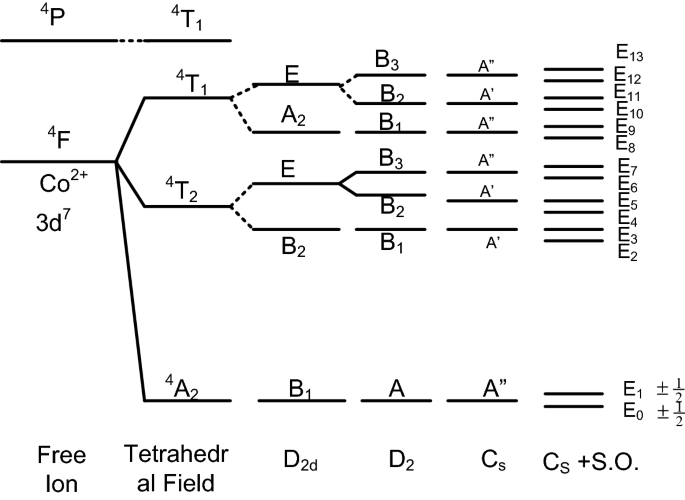
Another useful concept is the fictitious ‘spin’ associated with the fictitious ‘spin’ operator S′, which is selected artificially to describe a particular subset of N (N < Nt) distinct lowest-lying energy levels of a paramagnetic ion, out of the total manifold of Nt energy levels [3, 25,26,27]. This subset of energy levels is regarded as equivalent to a ‘spin’ multiplet of a fictitious spin operator S′, which is characterized by the ‘spin’ quantum number S′ and the magnetic quantum number MS′ (− S′ ≤ MS′ ≤ + S′). Important point is that the fictitious ‘spin’ S′ is ascribed in such a way that the multiplicity (2 S′ + 1) is equal to the number of the selected energy levels N. Conversely, the fictitious spin quantum number S′ equals to (N-1)/2. This definition implies that the fictitious spin quantum number S′ has, in fact, no direct relationship with that for the electronic spin S or the effective spin \(\tilde{S}\) of a paramagnetic ion (or spin system) in question. Hence, the natures of the spin operators S, S̃, and S′ are quite different from each other [3, 25,26,27]. Note that the fictitious ‘spin’ is not a purely mathematical concept. The major reason for introduction of a fictitious ‘spin’ is the experimental accessibility of transitions only between a limited number of low-lying states. The distinction between the three notions discussed above, i.e., S, S̃, and S′, is depicted in Fig. 2 for the case of Co2+(3d7) ions.
2.3 Forms of Spin Hamiltonian Suitable for Spin 3/2 and ½
For completeness, we provide the explicit SH forms suitable for the Co2+(3d7) ions in crystals. The distinction between the physical, effective, and fictitious quantities [3, 25,26,27,28] discussed in Sect. 2.2 is crucial for the present considerations. For the fictitious spin S′ = 1/2 system with a nuclear I = 7/2—like, e.g., the 59Co isotope, EMR spectra may be described by a general (triclinic) SH consisting of the Zeeman electronic (Ze) term and the hyperfine interaction term [1,2,3, 13,14,15,16,17,18]:
where μB is the Bohr magneton; g and A are the Zeeman and hyperfine structure tensors, respectively. For an S′ = 1/2 Kramers doublet at distorted tetragonal or lower symmetry sites in crystals, an orthorhombic-like SH form applies:
where as, for pure axial symmetry, gx= gy and Ax= Ay apply. The properties of the SH in Eq. (1) and (2) and the pertinent axes systems used in EMR studies have been discussed in [10].
For paramagnetic system with the effective spin 1 ≤ S̃ ≤ 3/2 general (triclinic) SH [3, 25,26,27,28]:
For S̃ =3/2 only the second rank ZFS terms exist, which for orthorhombic and lower symmetry may be expressed in the PAS of the second-rank ZFS terms [3, 25,26,27,28]:
A note of caution is pertinent concerning terminological confusion between key notions occurring in the literature. The generic A = B confusion denotes the cases pertaining to incorrect referral to the quantities associated with the notion B (e.g., ZFS) by the names of the quantities associated with the notion A (e.g., CF). The quantities may comprise Hamiltonians, eigenfunctions, energy-level splitting, or associated parameters. Such terminological confusion arises due to disregarding the well-accepted meaning of the given notions: A and B. The most common form is the CF = ZFS confusion, whereas the inverse ZFS = CF confusion has crept into literature in recent years [3, 25,26,27, 29, 30]. Other types of terminological confusion have been identified [3], including the MA = ZFS confusion between the notion ‘magnetic anisotropy (MA)’ (including related notions: magnetic anisotropy energy (MAE) and single-ion anisotropy (SIA)) and ZFS. This confusion occurs mainly in magnetism literature as outlined in the reviews in [31, 32] and more recently in studies of magnetic adatoms on surfaces [33].
Concerning the relations between the SH in Eq. (1) and (2) and that in Eq. (3), we note that for the effective spin Hamiltonian, the ZFS magnitude does not affect the g-tensor at all. However, for the fictitious spin S′ = 1/2 Hamiltonian, the g’-tensor is indeed related to the effective spin ZFS parameters, see, e.g., [34, 35]. On the other hand, the symmetry of the g- and g’-tensors as well as the ZFS tensor is determined by symmetry of the system and their explicit forms depend on the selected axes in which these tensors are expressed.
3 The High-Spin (HS) Co2+ (S = 3/2) Cases
Structural and magnetic properties of transition-metal complexes of pyridine N-oxide, including cobalt(II) ions in octahedral surroundings have been reviewed by Carlin and De Jongh [36]. This review provides an excellent background for the crystal field effects and paramagnetic behavior of Co2+ ions. Although cobalt(II) was described as ‘(3d7; L = 3; S = 3/2)’, i.e., an ion with the electronic spin S = 3/2, all systems have been described by the ‘effective’ spin S = ½ (so as discussed above, more properly the name fictitious ‘spin’ S′ = ½ should be used [3, 25,26,27,28]).
The reviews by Boča [20, 37] provide background CF theory for the HS Co2+ (S = 3/2) in regular octahedron and compressed tetragonal bipyramid with the ground state 4A2g, including the respective microscopic spin Hamiltonian (MSH) expressions for the ZFS parameter D and the gz factor as well as illustrative energy level diagrams in Fig. 3.
The spin levels for ions Co2+ (S̃= 3/2) at tetrahedral sites versus magnetic field B (||the c-axis) with the ZFS parameter D > 0 and D < 0; adapted from [37]
For the Co(II) complexes in tetrahedral geometry with the non-degenerate ground CF state 4A2(F), the ZFS parameter D typically adopts sizable values of +10 cm−1, whereas the g factors are larger than 2.0 and anisotropic [37]. The minimum and maximum ranges of D values (in cm−1) for Co2+ (S = 3/2) in mononuclear complexes derived from magnetic susceptibility (MS) data are listed in Table 27 of [37] as − 38 and + 73 for the coordination number CN = 5 and 4, whereas as + 25 and + 83 for the CN = 6 (see also Table 39 of [37]). The high-spin (HS) Co2+ (S = 3/2) cases identified in literature may be categorized into the groups by suitable ranges of |D| values. For the illustrative examples presented in Table 1, we tentatively adopt the ranges (in cm−1): small (up to 20) [38,39,40,41,42], moderate (20–50), large (50–100) [43, 44], and very large (over 100) [44]; for other references see, also Table 27 and 39 of [37]. Note that the ZFS Hamiltonian in Eqs. (3)–(5) employed for description of the EMR spectra for these cases actually pertains to the effective S̃ = 3/2 and not the (total) electronic spin S = 3/2.
Analysis of data in Table 1 indicates that even for small ZFS cases, HFM–EMR techniques [41, 42, 45, 46] may be very useful. However, for very large ZFS cases, these advanced techniques alone may not provide direct detection of the EMR transitions. Then, the ZFS parameters may be obtained from combined EMR and magnetic susceptibility measurements [44].
3.1 The Co2+ Ions with the Spin 3/2 in the Four- and Eightfold Coordination
The energy level diagram suitable for these cases is shown in Fig. 4. For the Co2+(3d7) ions at the tetrahedral (fourfold coordination) [or ‘cubic’ (eightfold coordination)] sites in crystals, there is splitting of the ground 4F multiplet under the action of the CF into the ground orbital singlet 4A2(F), and two orbital triplets 4T2(F) and 4T1 (F). Provided that the 4A2(F) singlet (or equivalent one in lower symmetry CF, see Fig. 4) is well separated from the next triplet 4T2(F) states, the microscopic SH approach [1,2,3, 13,14,15,16,17,18,19,20, 37] yields further splitting of the ground spin states due to the action of the spin-orbit coupling (SOC). This splitting may be calculated either by full diagonalization of the physical Hamiltonian or by perturbation methods. Thus, so-obtained splitting constitutes the physical ZFS (as in Fig. 3), i.e., equivalently the fine structure, which can be subsequently described by an effective SH [3, 25, 26].
In this case, the electronic spin S = 3/2 has the same value as the effective spin \(\tilde{S}\) = 3/2 within the ground spin states | \(\tilde{S}\) = 3/2, \(\tilde{M}_{S}\) = ± ½, ± 3/2 > . The transitions observable by EMR for the Co2+ ions at tetrahedral sites depicted in Fig. 1 depend on the sign and strength of the axial ZFS parameter D in Eq. (1). Importantly, if the resulting ZFS of the ground 4A2(F) states for the cases in question is very large, the lowest spin doublet state, i.e., \(\tilde{M}_{S}\) = ½ or \(\tilde{M}_{S}\) = 3/2, may only be observed by spectroscopic techniques such as EMR at low temperatures. Then, the EMR spectra may be described by the fictitious spin S′ = ½ yielding specific Co2+ (S′ = ½) cases. The data on the principal g- and A values and the ZFS parameters extracted from recent EMR and related studies as well as key older ones concerning the Co2+(S′ = 1/2) ions in the distorted tetrahedral coordination are collected in Table 2.
The above survey of the ground-state assignments and illustrative examples of the Co2+(S̃ = 3/2) and Co2+(S′ = ½) cases provided in this Section may help in better understanding of the Co2+ ions local environment in various systems, including low-symmetry cases. Detailed survey and analysis of both cases will be provided in a full review, with focus on most recent reports and HFM–EMR studies, as, e.g., on (Ph4P)2[Co(SPh)4] by Suturina et al. [50].
4 Summary and Conclusions
The Co2+(3d7) ions in crystals may occur in one of the two ‘configurations’ (or ‘states’): HS = the high-spin (S = 3/2) or LS = the low-spin (S = ½) configuration. For the tetrahedral (fourfold) and ‘cubic’ (eightfold) coordination, in the intermediate crystal field (CF), the ground state is 4A2g(F) with the high spin (S̃ = 3/2). For the TH coordination in the strong CF, the arrangement of the d-electron spins is somewhat controversial and several inconsistencies must be clarified. Survey of EMR studies of Co2+ systems has enabled systematic categorization of the origin of the ground states observed for Co2+ ions. Distinctions between the cases described by the effective spin S̃ = 3/2 (and S̃ = ½) and those by the fictitious ‘spin’ S′ = ½ have been elucidated. Implications of these distinctions for HMF–EMR measurements have been considered.
References
A. Abragam, B. Bleaney, Electron Paramagnetic Resonance of Transition Ions (Clarendon Press, Oxford, 1970)
S. K. Misra (ed.), Multifrequency Electron Paramagnetic Resonance (Wiley-VCH, Weinheim, 2011); Erratum, S. K. Misra, C. Rudowicz, http://www.wiley-vch.de/publish/dt/books/ISBN3-527-40779-0/. Accessed 15 Oct 2018.
C. Rudowicz, M. Karbowiak, Coord. Chem. Rev. 287, 28–63 (2015)
D. Piwowarska, P. Gnutek, C. Rudowicz, Book of Abstracts, p. 61. ISBN:978-83-60043-34-9 (Poster P19).
T. Sakurai, A. Taketani, A. Tomita, S. Okubo, H. Ohta, Y. Uwatoko, Rev. Sci. Instrum. 78, 65107 (2007). (6 pp)
T. Sakurai, K. Fujimoto, R. Goto, S. Okubo, H. Ohta, Y. Uwatoko, J. Magn. Reson. 223, 41–45 (2012)
T. Sakurai, K. Fujimoto, R. Matsui, K. Kawasaki, S. Okubo, H. Ohta, K. Matsubayashi, Y. Uwatoko, H. Tanaka, J. Magn. Reson. 259, 108–113 (2015)
M. Murrie, Chem. Soc. Rev. 39, 1986–1995 (2010)
V.P. Solnstev, E.G. Tsvetkov, A.I. Alimpiev, R.I. Mashkovtsev, Phys. Chem. Minerals 31, 1–11 (2004)
D. Piwowarska, A. Ostrowski, I. Stefaniuk, S.M. Kaczmarek, C. Rudowicz, Opt. Mater. 35, 2296–2302 (2013)
I. Stefaniuk, C. Rudowicz, P. Gnutek, A. Suchocki, Appl. Magn. Reson. 36, 371–380 (2009)
Z.Y. Yang, Y. Hao, C. Rudowicz, Y.Y. Yeung, J. Phys. 16, 3481–3494 (2004)
J.E. Wertz, J.R. Bolton, Electron Spin Resonance Elementary Theory and Practical Applications (McGraw-Hill, New York, 1972)
J.A. Weil, J.R. Bolton, J.E. Wertz, Electron Paramagnetic Resonance Elemental Theory and Practical Applications (Wiley, New York, 1994)
J.A. Weil, J.R. Bolton, Electron Paramagnetic Resonance Elemental Theory and Practical Applications (Wiley, New York, 2007)
S. Altshuler, B.M. Kozyrev, Electron Paramagnetic Resonance in Compounds of Transition Elements (Wiley, New York, 1974)
F.E. Mabbs, D. Collison, Electron Paramagnetic Resonance of d Transition-Metal Compounds (Elsevier, Amsterdam, 1992)
J.R. Pilbrow, Transition-ion Electron Paramagnetic Resonance (Clarendon Press, Oxford, 1990)
M. Wildner, M. Andrut, C. Rudowicz, in Spectroscopic Methods in Mineralogy-European Mineralogical Union Notes in Mineralogy, vol. 6, ed. by A. Beran, E. Libowitzky (Eötvös University Press, Budapest, 2004) Ch. 3, 93–143.
R. Boča, Struct. Bonding (Berlin) 117, 1–264 (2006)
D.J. Newman, B.Ng (eds.) in Crystal Field Handbook (Cambridge University Press, Cambridge, 2000) Ch. 5, pp 83–119.
B.N. Figgis, M.A. Hitchman, Ligand Field Theory, its Applications (Wiley-VCH, New York, 2000)
R.B. Burns, in Mineralogical Applications of Crystal Field Theory, 2nd edn. (Cambridge University Press, Cambridge, 1993)
C. Görller-Walrand, K. Binnemans, in Handbook on the Physics and Chemistry of Rare Earths, vol. 23, ed. by J.K.A. Gschneidner, L. Eyring (Elsevier, Amsterdam, 1996) Ch. 155, pp. 67–81.
C. Rudowicz, Magn. Reson. Rev. 13, 1–89 (1987)
C. Rudowicz, S.K. Misra, Appl. Spectrosc. Rev. 36, 11–63 (2001)
C. Rudowicz, H.W.F. Sung, Phys. B 300, 1–26 (2001)
C. Rudowicz, P. Gnutek, Phys. B 404, 3582–3593 (2009)
C. Rudowicz, M. Karbowiak, Phys. B 451, 134–150 (2014)
C. Rudowicz, M. Karbowiak, Phys. B 456, 330–338 (2015)
C. Rudowicz, Phys. B 403, 1882–1897 (2008)
C. Rudowicz, Phys. B 403, 2312–2330 (2008)
C. Rudowicz, K. Tadyszak, Polyhedron 127, 126–134 (2017)
M.C. Chen, J.O. Artman, Phys. Rev. B 187, 723 (1969)
D. Piwowarska, S.M. Kaczmarek, P. Gnutek, C. Rudowicz, Acta Phys. Polon. A 132, 73–76 (2017)
R.L. Carlin, L. De, J. Jongh, Chem. Rev. 86, 659–680 (1986)
R. Boča, Coordin. Chem. Rev. 248, 757–815 (2004)
J. Derouet, L. Beaury, P. Porcher, P.J. Deren, J. Alloy. Compounds 300–301, 242–253 (2000)
D. Nelson, L.W. Haar, Inorg. Chem. 32, 182–188 (1993)
Y. Chiba, N. Kontani, M. Date, J. Phys. Soc. Jpn. 57, 1449–1450 (1988)
K. Krzystek, S.A. Zvyagin, A. Ozarowski, A.T. Fiedler, T.C. Brunold, J. Telser J. Am. Chem. Soc. 126, 2148–2155 (2004)
J.M. Zadrozny, J. Liu, N.A. Piro, C.J. Chang, S. Hill, J.R. Long, Chem. Comm. 48, 3897–4020 (2012)
J.D. Garrity, B. Bennett, M.W. Crowder, Biochem. 44, 1078–1087 (2005)
K. Fukui, N. Kojima, H. Ohya-Nishiguchi, N. Hirota, Inorg. Chem. 31, 1338–1344 (1992)
J. Telser, J. Krzystek, A. Ozarowski, J. Biol. Inorg. Chem. 19, 297–318 (2014)
J. Telser, A. Ozarowski, J. Krzystek, Electron Paramag. Reson. 23, 209–263 (2012)
A.A. Verberckmoes, B.M. Wechuysen, R.A. Schoonheydt, Micro. Meso. Mat. 22, 165–178 (1999)
A. Edgar, J. Phys. C: Solid State Phys. 9, 4304–4316 (1976)
M.D. Sturge, F.R. Merritt, J.C. Hensel, J.P. Remeika, Phys. Rev. 180, 402–412 (1969)
E.A. Suturina, J. Nehrkorn, J.M. Zadrozny, J. Liu, M. Atanasov, T. Weyhermuller, D. Maganas, S. Hill, A. Schnegg, E. Bill, J.R. Long, F. Neese, Inorg. Chem. 56, 3102–3118 (2017)
Acknowledgements
This work was partially supported by the research grant no. 2016/21/B/ST4/02,064 from the Polish National Science Center. Thanks to the anonymous reviewers for helpful comments and bringing a pertinent reference to our attention.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
Open Access This article is distributed under the terms of the Creative Commons Attribution 4.0 International License (http://creativecommons.org/licenses/by/4.0/), which permits unrestricted use, distribution, and reproduction in any medium, provided you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made.
About this article
Cite this article
Piwowarska, D., Gnutek, P. & Rudowicz, C. Origin of the Ground Kramers Doublets for Co2+(3d7) Ions with the Effective Spin 3/2 Versus the Fictitious ‘Spin’ ½. Appl Magn Reson 50, 797–808 (2019). https://doi.org/10.1007/s00723-018-1080-4
Received:
Revised:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00723-018-1080-4