Abstract
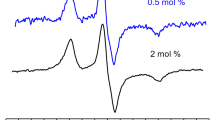
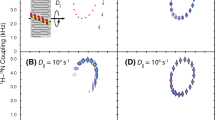
The ion channel-forming peptide antibiotic zervamicin A was studied in egg phosphocholin lipid membranes of large multilamellar vesicles (LMV) at 77 K. Continuous wave electron paramagnetic resonance (EPR) and electron spin echo envelope modulation (ESEEM) methods combined with site-specific electron spin labeling were used to study the aggregation and immersion depth of two analog molecules, i.e., each monolabeled either at the N- or C-terminal end of the helical molecule. Analysis of the shape of the EPR spectra indicates that zervamicin molecules form aggregates in which the dipolar interaction between the spin labels at the N-terminus is substantially larger than that between the labels at the C-terminus. The ESEEM method was used to study the interaction between the nitroxide radical spin labels of the zervamicin molecules and deuterium nuclei in LMV, which were prepared using a D2O buffer. It is established that the largest amplitude of deuterium modulation of the unpaired electron is observed for zervamicin molecules labeled at the N-terminus. Based on the analysis of the Fourier parameters of the deuterium modulated spectrum, a model of the immersion depth of the terminal ends of the zervamicin molecule in a lipid bilayer is formulated. All of the spin labels at the N-terminus are grouped at the lipid–water interface, whereas 60% of labels at the C-terminus are located at the lipid–water interface and 40% are more deeply inserted into the lipid bilayer.





Similar content being viewed by others
References
A.D. Milov, R.I. Samoilova, Yu.D. Tsvetkov, M. DeZotti, F. Formaggio, C. Toniolo, J.-W. Handgraaf, J. Raap, Biophys. J. 96, 3197–3209 (2009)
B. Bechinger, D.A. Skladnev, A. Ogrel, X. Li, E.V. Rogozhkina, T.V. Ovchinnikova, J.D.J. O’Neil, J. Raap, Biochemistry 40, 9428–9437 (2001)
A.D. Milov, Yu.D. Tsvetkov, E.Yu. Gorbunova, L.G. Mustaeva, T.V. Ovchinnikova, J.-W. Handgraaf, J. Raap, Chem. Biodiver. 4, 1243–1255 (2007)
F. Szoka Jr., D. Papahadjopoulos, Ann. Rev. Biophys. Bioeng. 9, 467–508 (1980)
J.-M. Fauth, A. Schweiger, L. Braunschweiler, J. Forrer, R.R. Ernst, J. Magn. Reson. 66, 74–85 (1986)
V.N. Parmon, A.I. Kokorin, G.M. Zhidomirov, Stable biradicals (Nauka, Moscow, 1980), p. 240
T.A. Balashova, Z.O. Shenkarev, A.A. Tagaev, T.V. Ovchinnikova, J. Raap, A.S. Arseniev, FEBS Lett. 466, 333–336 (2000)
A.D. Milov, R.I. Samoilova, Yu.D. Tsvetkov, M. DeZotti, C. Toniolo, J. Raap, J. Phys. Chem. B 112, 23469–23472 (2008)
R. Bartucci, R. Guzzi, D. Marsh, L. Sportelli, Biophys. J. 84, 1025–1030 (2003)
D.A. Erilov, R. Bartucci, R. Guzzi, A.A. Shubin, A.G. Maryasov, D. Marsh, S.A. Dzuba, L. Sportelli, J. Phys. Chem. B 109, 12003–12013 (2005)
R. Bartucci, D.A. Erilov, R. Guzzi, L. Sportelli, S.A. Dzuba, D. Marsh, Chem. Phys. Lipids 141, 142–157 (2006)
E.S. Salnikov, D.A. Erilov, A.D. Milov, Yu.D. Tsvetkov, C. Peggion, F. Formaggio, C. Toniolo, J. Raap, S.A. Dzuba, Biophys. J. 91, 1532–1540 (2006)
S.A. Dikanov, Yu.D. Tsvetkov, Electron spin echo envelope modulation (ESEEM) spectroscopy (CRC Press, Boca Raton, 1992), p. 412
A.D. Milov, R.I. Samoilova, A.A. Shubin, Yu.A. Grishin, S.A. Dzuba, Appl. Magn. Reson. 35, 73–94 (2008)
I.L. Karle, J. Flippen-Anderson, M. Sukumar, P. Balaram, Proc. Natl. Acad. Sci. USA 84, 5087–5091 (1987)
T.N. Kropacheva, E.S. Salnikov, H.-H. Nguyen, S. Reissmann, Z.A. Yakimenko, A.A. Tagaev, T.V. Ovchinnikova, J. Raap, Biochim. Biophys. A 1715, 6–18 (2005)
Acknowledgments
This work was supported by the Russian Grant for Scientific Schools (551-2008.3) and the Netherlands Organization of Scientific Research (NWO/RFBR 0.47.017.034).
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Milov, A.D., Samoilova, R.I., Shubin, A.A. et al. Self-Aggregation and Orientation of the Ion Channel-Forming Zervamicin IIA in the Membranes of ePC Vesicles Studied by cw EPR and ESEEM Spectroscopy. Appl Magn Reson 38, 75–84 (2010). https://doi.org/10.1007/s00723-009-0104-5
Received:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00723-009-0104-5