Abstract
The crystal structure of uralolite, Ca2Be4(PO4)3(OH)3·5H2O, from the spodumene deposit of Weinebene, Carinthia, Austria, has been refined with X-ray single crystal data gathered on a CCD diffractometer. Uralolite is monoclinic, space group P21/n, a = 6.553(1), b = 16.005(3), c = 15.979(3) Å, β = 101.63(1)°, V = 1641.5(5) Å3. While previously only isotropic displacement parameters and no hydrogen atom positions were reported for uralolite, now anisotropic displacement parameters were used for non-hydrogen atoms and hydrogen atoms were located and refined yielding R1 = 0.038 for 3909 observed reflections. Uralolite is built up from corrugated layers [Be4(PO4)3(OH)3]4- parallel to (010), which contain Z-shaped groups of four BeO4 tetrahedra sharing corners via three OH groups and are further crosslinked by PO4 tetrahedra. Two Ca atoms in Ca(Ophosphate)5(H2O)2 coordination and an interstitial water molecule link these layers along [010]. The OH groups and the Ca-bonded H2O molecules are all involved in hydrogen bonds with O···O distances of 2.780(2) − 3.063(2) Å and O-H···O angles of 150(1) − 179(1)° excluding a bifurcated bond. The interstitial water molecule displays a distorted tetrahedral environment of O atoms and accepts and donates each two hydrogen bonds. The crystal structure exhibits a C2/c pseudosymmetry for the [Be4(PO4)3(OH)3]4- layers and the Ca atoms. However, the disposition of the water molecules and an asymmetric hydrogen bond pattern involving OH groups as well as H2O molecules are decisive for the lowering of the symmetry of the structure to the true space group P21/n.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Uralolite is a rare hydrated calcium beryllium phosphate occurring in fibrous aggregates of minute acicular to lath-like crystals in fractures of the Weinebene spodumene pegmatites, Carinthia, Austria. A description of this deposit based on extensive pre-mining exploration was reported by Göd (1989). A rich variety of about 30 primary and more than 30 secondary minerals and their paragenetic relationships have been reported from this deposit (Niedermayr and Göd 1992; Taucher et al. 1992, 1994). The presently known Be-bearing minerals of this deposit comprise primary beryl, Be3Al2Si6O12, and secondary/tertiary uralolite, Ca2Be4(PO4)3(OH)3·5H2O, weinebeneite, CaBe3(PO4)2(OH)2·4H2O (type locality; Walter 1992), roscherite Ca2Mn2 +5Be4(PO4)6(OH)4·6H2O, hydoxylherderite, CaBe(PO4)(OH), and bavenite, Ca4Be2Al2Si9O26(OH)2 (Taucher et al. 1992; Niedermayr and Göd 1992). The deposit is currently of significant economic interest for the mining of spodumene ore and production of lithium ion battery grade Li2CO3 and LiOH·2H2O (European Lithium Ltd 2020). Only nine further occurrences of uralolite are known at present (Mindat.org 2022) and all of them with very small amounts in pegmatitic settings (Grigoriev 1964; Dunn and Gaines 1978). The properties and crystal structure of uralolite from Weinebene were previously reported by Mereiter et al. (1994). This work was based on single crystal diffraction data recorded with a Philips PW1100 four-circle diffractometer and a scintillation probe as point detector. With space group P21/n, a = 6.550(1), b = 16.005(3), c = 15.969(3) Å, β = 101.64(2)°, Z = 4 Ca2Be4(PO4)3(OH)3·5H2O, it yielded R1 = 0.058 for 1651 observed out of 2878 independent reflections (θmax = 25°, MoKα radiation, 117 refined parameters). Due to a pronounced pseudosymmetry of the crystal structure with space group C2/c for a zellengleiche pseudostructure, hkl reflections with h + k = 2n + 1 were systematically weak. In order to overcome parameter correlation effects only isotropic displacement parameters were applied in this work and hydrogen atoms could not be located. With this situation in mind, it was considered worthwhile to refine the crystal structure of uralolite with CCD detector diffraction data using a larger data set with more h + k = 2n + 1 reflections in order to apply anisotropic displacement parameters for non-hydrogen atoms and to locate the hydrogen atoms.
Experimental
Using a crystal preserved from the previous study (Mereiter et al. 1994), X-ray diffraction data were collected with a Bruker Smart CCD diffractometer and graphite monochromatized MoKα radiation, λ = 0.71073 Å, from a sealed tube. A crystal measuring 0.28 × 0.08 × 0.02 mm was applied to collect six ω-scan frame sets covering a full sphere of the reciprocal space up to θmax = 30.1°. The raw data were integrated and processed with the program SAINT (Bruker 1999) and were then corrected for absorption with the multi-scan method using program SADABS (Bruker 1999). The resulting 38377 data were then merged to 4823 unique reflections (Rmerge = 0.050). Structure refinement was carried out with program SHELXL (Sheldrick 2015). Anisotropic temperature factors for non-hydrogen atoms were applied before a difference Fourier synthesis revealed clearly 12 of the 13 expected hydrogen atoms. The missing hydrogen atom, belonging to the interstitial water molecule of O5w, was obscured by relatively large anisotropic displacement effects of this oxygen atom, but could be reasonably inferred by geometric considerations (distorted tetrahedral environment of O5w by two H-bond donors and two adequate H-bond acceptors). All hydrogen atoms were then included in the refinement, at first unrestrained (O-H = 0.78(4) to 1.00(6) Å, mean value 0.86 Å; Uiso = 0.03–0.15 Å2), and then with geometric restraints: The O-H distances were fixed at 0.85 Å and H-O-H angles at 108° using hard DFIX restraints. The isotropic displacement parameters of the H atoms were divided into three groups – hydroxyl groups, water molecules of O1w to O4w, and water molecule of O5w – and each group was refined with a common Uiso. These procedures were considered as useful in obtaining the most reliable and consistent information on hydrogen bonding. A check of the occupancy of the interstitial water molecule of O5w gave a population factor of 0.99(1) indicating that there is no deficiency. The final refinement converged at R1 = 0.0380 for 3909 Fo > 4σ(Fo). Crystallographic data are summarized in Table 1, atomic parameters are given in Tables 2 and 3. Selected bond lengths and bond angles are presented in Table 4, and hydrogen bond data are reported in Table 5. A crystallographic information file (CIF) with structure factors is available as electronic supplementary material. Structural graphics was generated with programs MERCURY (Macrae et al. 2006) and DIAMOND (Brandenburg 2012).
Discussion
The present study confirms the previous structure determination of Mereiter et al. (1994) but provides now very satisfactory anisotropic displacement parameters for non-hydrogen atoms, hydrogen atom positions, and an improvement in the precision of atomic positions and bond lengths. Although the e.s.d.s for bond lengths to Ca, Be, and P are reduced to about one third of their former values (Mereiter et al. 1994), the changes in geometric parameters are small and do not warrant a detailed comparison with the earlier values (Table 4). An important result is the clarification of the hydrogen bonds in uralolite, which play a distinctive role in the structure and its symmetry.
[Be4(PO4)3(OH)3]4- layers
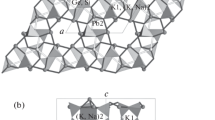
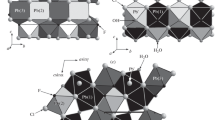
The structure of uralolite is based on corrugated layers of composition [Be4(PO4)3(OH)3]4- which extend parallel to (010) at y = ¼ and ¾ of the monoclinic unit cell, and which are mutually linked via two Ca(H2O)22+ fragments and an additional interstitial water molecule (Fig. 1). As shown in Fig. 2 the [Be4(PO4)3(OH)3]4- layer contains a unique Z-shaped group of four BeO4 tetrahedra crosslinked by PO4 groups. The four independent Be atoms are linked via three OH groups. Be2 and Be3 are each bonded to two OH groups and two PO4 oxygen atoms. Be1 and Be4 in turn are each bonded to one OH group and three PO4 oxygen atoms. Of the three independent PO4 groups, those of P1 and P2 link three Be and have each one oxygen atom (O4, O8) in terminal position while those of P3 link four Be. Thus, in terms of the links between tetrahedra, all four BeO4 and one PO4 tetrahedra are four-connected and two PO4 are three-connected. The [Be4(PO4)3(OH)3]4- layer (Fig. 1a) contains following tetrahedral rings: Three-membered BeBeP, four-membered BeBeBeP, four-membered (BeP)2, six-membered (BeBeP)2 and eight-membered (BeP)4 rings. As pointed out previously (Mereiter et al. 1994), and remaining unchanged ever since, the four-membered rings BeBeBeP in uralolite are unique among beryllophosphates, arsenates and silicates as well as the tetrahedral architecture of the layer among silicates (Liebau 1985; Hawthorne and Huminicki 2002). For a comparison of sheet topologies in beryllium minerals including uralolite, see Huminicki and Hawthorne (2002). Bond lengths and bond angles in the BeO4 and PO4 tetrahedra show usual variations with Be-O ranging from 1.600(3) to 1.663(3) Å, mean value 1.632 Å, and P-O ranging from 1.5071(17) to 1.5548(16) Å, mean value 1.535 Å. In their review on the crystal chemistry of beryllium, Hawthorne and Huminicki (2002) give Be-O = 1.633 Å as grand mean tetrahedral Be-O bond distance. Bond angles O-Be-O are in the range 100.53(17)-116.62(19)°, and O-P-O angles 105.13(9)-112.50(10)°, where in both cases the lowest values represent shared edges between the Ca polyhedra and BeO4 as well as PO4 tetrahedra (Fig. 3). The Be-O-Be bond angles of O1h and O2h (115.60(17) and 117.20(17)°) are both involved in tetrahedral BeBeP rings. They are notably smaller than that of O3h (126.90(16)°), which bridges the two inner BeO4 tetrahedra and is part of two BeBeBeP rings (Fig. 2).
Ca coordination
The coordination of the two independent Ca atoms and how they connect the [Be4(PO4)3(OH)3]4- layers in detail is shown in Fig. 3. Both Ca atoms are seven coordinated and form CaO5(H2O)2 polyhedra, where the five phosphate oxygen atoms adopt a roughly equatorial arrangement about Ca and the two water molecules are in approximately axial trans-disposition. Each CaO5(H2O)2 polyhedron shares edges with one BeO4 and one PO4 tetrahedron and one edge with its centrosymmetric counterpart. The Ca-O bond distances vary from 2.2431(17) to 2.5448(17) Å and have mean values of 2.414 and 2.423 Å for Ca1 and Ca2, respectively. A mean bond length of 2.448(133) Å was reported for 7-coordinated Ca (Gagné and Hawthorne 2016). In each polyhedron the shortest and longest Ca-O bonds are to the two oxygen atoms that are shared by two Ca, O4 and O4' in case of Ca1, and O8 and O8' in case of Ca2 (Fig. 3; atoms with primed labels are used to distinguish them from their inversion related equivalents). Despite the apparent similarity of the environments of Ca1 and Ca2, their water molecules show significant differences in their positions relative to Ca, in H2O orientations, and in hydrogen bonding. At comparable distances between Ca1-Ca1' = 3.954(1) Å and Ca2-Ca2' = 3.802(1) Å, the separation between O1w and O2w' is 3.668(2) Å, a value much larger than between O3w and O4w', 2.831(2) Å, which represents a hydrogen bond (Fig. 3) addressed below.
OH groups, H2O molecules and hydrogen bonding
The hydrogen bond scheme discussed previously (Mereiter et al. 1994) was found to be largely correct except for two bonds involving O1w and O5w. The hydrogen bonds found in the present investigation are listed in Table 5. A graphic representation of the entire hydrogen bond system is included in Fig. 1b. The three OH groups of the [Be4(PO4)3(OH)3]4- layer adopt distorted tetrahedral coordination figures by two Be, one donated hydrogen bond and one accepted hydrogen bond. Most intriguing is the hydrogen bond O2h-H2h···O1h (O2h···O1h = 2.871(2) Å) within the Be4 cluster shown in Fig. 2. It leads to an asymmetry in the Be4 cluster because it forces O1h-H1h to adopt a hydrogen bond with an external acceptor O3w (O1h···O3w = 2.875(2) Å), whereas O2h in turn becomes acceptor of a hydrogen from water molecule O1w (O1w···O2h = 2.784(2) Å). The third OH group of O3h is donor and acceptor of hydrogen bonds to the interstitial water molecule of O5w and from the Ca1-bonded water molecule of O1w, respectively. Of the four Ca-bonded water molecules O1w stands out by having the shortest Ca-Ow bond (Ca1-O1w by ~ 0.1 Å shorter than the remaining three Ca-Ow bonds), by being no hydrogen bond acceptor, and by having an approximately planar coordination by Ca1 and three hydrogen bond acceptors, with O2h forming a linear and with O3 and O12 a bifurcated hydrogen bond. The remaining three Ca-bonded water molecules have longer Ca-Ow bonds (≈ 2.45 Å) and show tetrahedral environments by one Ca, one hydrogen bond donor (O···O ≈ 2.8 Å) and two hydrogen bond acceptors (O···O ≈ 2.78–2.91 Å). The interstitial water molecule O5w accepts two clearcut hydrogen bonds from O3h and O2w (O···O ≈ 2.95 and 2.78 Å) but has troubles with a pair of suitable acceptor atoms. According to the structure refinement the acceptors are O9 and O12 forming an edge of the PO4 tetrahedron of P3, but the H-bonds are strongly bent and weak (Table 5). This water molecule has the notably largest displacement parameters of the structure (Table 2) and appears to rattle around modestly in a closed structural cavity. It was proven that this water molecule position is fully occupied, but its hydrogen bond parameters d(H···A) and <(DHA) (Table 5) are certainly inaccurate. Nevertheless a hydrogen bond interaction with O1w (O5w···O1w = 3.15 Å) can be ruled out, particularly by taking the bonding situation of O1w into account, especially the short Ca1-O1w bond.
Pseudosymmetry
The pseudosymmetry of the crystal structure of uralolite has been pointed out previously (Mereiter et al. 1994). In Fig. 1a it can be noted that in addition to the symmetry elements of space group P21/n, there are twofold pseudo-rotations parallel to [010] at x,z = 0,¼ (passing near P3) and ½,¼ (passing near O3h, the corner shared between Be2 and Be3 tetrahedra), and pseudo-inversions at x,y,z = ¼,¼,0 (relating approximately for instance adjacent P1 and P2) and x,y,z = ¾,¼,0 (relating approximately adjacent Ca1 and Ca2 for instance). By modest shifts of their atoms the P21/n structure can thus be transformed into a zellengleiche structure with pseudo-space group C2/c. Whereas deviations from space group symmetry C2/c are small for Ca atoms and [Be4(PO4)3(OH)3]4- layers disregarding hydrogen atoms (0.06–0.22 Å), they are significant for all water molecules, hydrogen atoms, and hydrogen bonds (0.42–0.92 Å for O1w through O5w). A good example for the latter deviations can be seen in Fig. 1b for the water molecules of O2w and O4w near y,z = ½,¼ which differ in height by 0.72 Å along [010], and much more so with regard of the positions of their hydrogen atoms and hydrogen bonds. Qualitatively, the same is valid for the pair O1w and O3w (corresponding height difference 0.65 Å). These features lead to the differences in Ca coordination outlined above and in Fig. 3. A simple proof for the pseudosymmetry is that the crystal structure can be refined in space group C2/c using only reflections with h + k = 2n, and with one Ca (Ca1), two Be (Be1, Be2), one Oh (O1h), one PO4 tetrahedron (P1, O1 through O4) and O9, O10 of P3 in general positions (Z = 8 in C2/c, Z = 4 in P21/n), whereas P3 and O3h adopt special positions on twofold axes. The water molecules O1w through O4w show up in half occupied split position pairs, and O5w near a twofold axis and also in a half-occupied general position. Using anisotropic displacement parameters for all atoms except water oxygen atoms and neglecting H atoms the structure can be refined in space group C2/c to R1 = 0.0403 for 2128 Fo > 4σ(Fo) (h + k = 2n only). The outlined features show that the entire system of hydrogen bonds starting with the asymmetry of the three Be-bonded OH groups and ending with all water molecules are decisive for the true space group symmetry P21/n of uralolite and its deviation from C2/c pseudosymmetry.
Structural relationships
Overviews on beryllium in earth sciences including beryllium minerals and their crystal chemistry have been given in the review volume 50 of the Mineralogical Society of America (Hawthorne and Huminicki 2002). Therefore, the subsequent discussion is restricted only to calcium beryllium phosphate minerals without further cations, namely hurlbutite, CaBe2(PO4)2, hydroxylherderite, CaBe(PO4)(OH), weinebeneite, CaBe3(PO4)2(OH)2·4H2O, uralolite, Ca2Be4(PO4)3(OH)3·5H2O, and fransoletite / parafransoletite, Ca3Be2(PO4)2(PO3OH)2·4H2O.
Hurlbutite, CaBe2(PO4)2, has a framework structure built up from alternating 4-connected BeO4 and PO4 tetrahedra forming (BeP)2, (BeP)3, and (BeP)4 tetrahedral rings with an architecture analogous to danburite, CaB2Si2O8, and related to feldspars like anorthite, CaAl2Si2O8 (Lindbloom et al. 1974). Ca is 7-coordinated with mean Ca-O = 2.469 Å disregarding a further oxygen at 3.09 Å. The CaO7 polyhedron shares edges with a BeO4 and a PO4 tetrahedron (not adjacent edges). The O-T-O angles (T = Be, P) corresponding to the shared edges are the smallest in this structure (102.8 and 103.9°), just like in uralolite.
Hydroxylherderite, CaBe(PO4)(OH), has a layer structure built up from alternating 3-connected BeO3(OH) and PO4 tetrahedra forming (BeP)2 and (BeP)4 tetrahedral rings where the Be-bonded OH group and a phosphate oxygen atom are in terminal positions. All hydroxylherderites investigated so far have a significant OH by F substitution leading to ideal herderite CaBe(PO4)F (Harlow and Hawthorne 2008). Ca is 8-coordinated in the shape of a square antiprism with a mean bond length of Ca-O ≈ 2.50 Å, where four of the ligands are two Be-bonded OH-groups and two terminal PO4 oxygen atoms. The polyhedron shares edges with two BeO4 tetrahedra, but not with PO4, and the corresponding O-Be-O bond angles are small (103–106°). Each CaO8 polyhedron shares edges with three adjacent Ca polyhedra.
Weinebeneite, CaBe3(PO4)2(OH)2·4H2O, at present still only known from the namesake occurrence (Walter 1992) and occurring there in paragenesis with uralolite (Taucher et al. 1992), bears some close relationships to the latter. It has a framework structure being built up from 4-connected BeO3(OH), BeO2(OH)2, and PO4 tetrahedra and contains BeBeP, (BeP)2, (BeBeP)2, and (BeP)4 tetrahedral rings. BeBeP rings are presently known only from uralolite, weinebeneite, moraesite, Be2(PO4)(OH)·4H2O (Merlino and Pasero 1992), and väyrynenite, (Mn,Fe)Be(PO4)(OH,F) (Mrose and Appleman 1962; Huminicki and Hawthorne 2000). A most distinctive feature of weinebeneite is a quasi-linear group of three BeO4 tetrahedra linked by sharing vertices via two OH groups, i.e. Op3Be(OH)BeOp2(OH)BeOp3 where Op denote phosphate O atoms (Walter 1992). This unique trimer relates weinebeneite to the Z-shaped tetramer of BeO4 tetrahedra in uralolite. An infinite extension of the OH mediated link of BeO4 tetrahedra is present in the Be(PO4)(OH,F) chains of väyrynenite. The tetrahedral framework of weinebeneite contains large zeolite-like infinite pores extending along the a-axis, and these pores are occupied by Ca and four Ca-bonded water molecules.
Finally, fransoletite and its dimorph parafransoletite, Ca3Be2(PO4)2(PO3OH)2·4H2O (Kampf 1992), stand out by bearing OH groups not on BeO4 but on PO4 tetrahedra. Here 4-connected BeO4 and 3-connected PO4 tetrahedra (half of them bearing OH) form infinite chains of (BeP)2 rings crosslinked by CaO4(H2O)2 and CaO5(H2O)2 polyhedra.
References
Brandenburg K (2012) Program DIAMOND. Crystal Impact GbR, Bonn, Germany
Bruker (1999) Programs SMART, SAINT and SADABS. Bruker AXS Inc., Madison, Wisconsin, USA
Dunn PJ, Gaines RV (1978) Uralolite from the Dunton Gem Mine, Newry, Maine: A second occurrence. Min Record 9(2):99–100
European Lithium Ltd (2020) https://europeanlithium.com. Accessed 15 Sept 2020
Gagné OC, Hawthorne FC (2016) Bond-length distributions for ions bonded to oxygen: alkali and alkaline-earth metals. Acta Crystallogr B72:602–625
Göd R (1989) The spodumene deposit at “Weinebene”, Koralpe, Austria. Mineral Deposita 24:270–278
Grigoriev NA (1964) Uralolite, a new mineral. Zap Vses Mineralog Obshch 93(2):156–162
Harlow GE, Hawthorne FC (2008) Herderite from Mogok, Myanmar, and comparison with hydroxyl-herderite from Ehrenfriedersdorf, Germany. Am Mineral 93:1545–1549
Hawthorne FC, Huminicki DMC (2002) The crystal chemistry of beryllium. Pp. 333–403 in: Beryllium: Mineralogy, Petrology, and Geochemistry (E.S. Grew, editor). Reviews in Mineralogy and Geochemistry, 50. Mineralogical Society of America and the Geochemical Society, Chantilly, Virginia, USA
Huminicki DMC, Hawthorne FC (2000) Refinement of the crystal structure of väyrynenite. Can Mineral 38:1425–1432
Huminicki DMC, Hawthorne FC (2002) Refinement of the crystal structure of aminoffite. Can Mineral 40:915–922
Kampf AR (1992) Beryllophosphate chains in the structures of fransoletite, parafransoletite, and ehrleite and some general comments on beryllophosphate linkages. Am Mineral 77:848–856
Liebau F (1985) Structural chemistry of silicates. Structure, bonding, and classification. Springer-Verlag, Berlin Heidelberg New York Tokyo
Lindbloom JT, Gibbs GV, Ribbe PH (1974) The Crystal Structure of Hurlbutite: A comparison with danburite and anorthite. Am Mineral 59:1267–1271
Macrae CF, Edgington PR, McCabe P, Pidcock E, Shields GP, Taylor R, Towler M, van de Streek J (2006) Mercury: visualization and analysis of crystal structures. J Appl Cryst 39:453–457
Mereiter K, Niedermayr G, Walter F (1994) Uralolite, Ca2Be4(PO4)3(OH)3·5H2O: new data and crystal structure. Eur J Mineral 6:887–896
Merlino S, Pasero M (1992) Crystal chemistry of beryllophosphates: The crystal structure of moraesite, Be2(PO4)(OH)·4H2O. Z Kristall 201:253–262
Mindat.org (2022) “Uralolite” https://www.mindat.org/min-4099.html; Accessed 24 Aug 2022
Mrose ME, Appleman DE (1962) The crystal structures and crystal chemistry of väyrynenite, (Mn,Fe)Be(PO4)(OH), and euclase, AlBe(SiO4)(OH). Z Kristall 117:16–36
Niedermayr G, Göd R (1992) Das Spodumenvorkommen auf der Weinebene und seine Mineralien. Carinthia II 182/102:21–35
Sheldrick GM (2015) Crystal structure refinement with SHELXL. Acta Cryst C71:3–8
Taucher J, Walter F, Postl W (1992) Mineralparagenesen in Pegmatiten der Koralpe. Teil 1. Die Lithium-Lagerstätte am Brandrücken, Weinebene, Koralpe, Kärnten. Die Minerale des feinkörnigen Spodumenpegmatits (MH-Pegmatit). Matrixx Mineralogische Nachrichten aus Österreich 1:23–72
Taucher J, Walter F, Postl W (1994) Mineralparagenesen in Pegmatiten der Koralpe. Teil 2. Die Lithium-Lagerstätte am Brandrücken, Weinebene, Koralpe, Kärnten. Die Minerale des grobkörnigen Spodumenpegmatits (AH-Pegmatit) sowie die Minerale der Pegmatitrandgesteine. Matrixx, Mineralogische Nachrichten aus Österreich 3:19–52
Walter F (1992) Weinebeneite, CaBe3(PO4)2(OH)2·4H2O, a new mineral species: mineral data and crystal structure. Eur J Mineral 4:1275–1283
Acknowledgements
The X-ray centre of TU Wien is acknowledged for providing access to the single-crystal X-ray diffractometer. The authors thank the two anonymous reviewers and the editors Manfred Wildner and Lutz Nasdala for their comments and suggestions.
Funding
Open access funding provided by TU Wien (TUW).
Author information
Authors and Affiliations
Corresponding author
Additional information
Editorial handling: M. Wildner
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic Supplementary Material
Below is the link to the electronic supplementary material
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Mereiter, K., Walter, F. A crystal structure refinement of uralolite, Ca2Be4(PO4)3(OH)3∙5H2O, from Weinebene, Austria. Miner Petrol 117, 181–189 (2023). https://doi.org/10.1007/s00710-022-00806-x
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00710-022-00806-x