Abstract
The crystal structures of synthetic hexagonal and orthorhombic Fe-cordierite polymorphs with the space groups P6/mcc and Cccm were refined from single-crystal X-ray diffraction data to R 1, hex = 3.14 % and R 1, ortho = 4.48 %. The substitution of the larger Fe2+ for Mg leads to multiple structural changes and an increase of the unit cell volumes, with a, c (hex) = 9.8801(16) Å, 9.2852(5) Å and a, b, c (ortho) = 17.2306(2) Å, 9.8239(1) Å, 9.2892(1) Å in the end-members. Furthermore Fe incorporation results in an increase of the volumes of the octahedra, although the diameters of the octahedra in direction of the c-axis decrease in both polymorphs. X-ray powder diffraction analysis indicates a high degree of Al/Si ordering in the orthorhombic polymorph, the Miyashiro distortion index is ~0.24. Estimations of site occupancies based on the determined tetrahedral volumes result in the following values for hexagonal Fe-cordierite: ~73 % Al for T1 and ~28 % Al for T2. For the first time Raman spectroscopy was performed on the hexagonal Fe-cordierite polymorph. In the hexagonal Fe-cordierite polymorph most Raman peaks are shifted towards lower wavenumbers when compared with the Mg-end-member.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Cordierite with the simplified formula (Mg, Fe)2Al4Si5O18*n(H2O, CO2) is a framework silicate whose structure consists of corner-sharing T2 tetrahedra forming six-membered rings which are crosslinked by additional T1 tetrahedra. Crystal structure and Al/Si-ordering of Mg-Fe-cordierites were investigated by various authors (e.g., Gibbs 1966; Langer and Schreyer 1969; Meagher and Gibbs 1977; Cohen et al. 1977; Hochella et al. 1979; Putnis 1980; Wallace and Wenk 1980; Armbruster and Bloss 1981; Putnis and Bish 1983; Gunter et al. 1984; Mirwald and Kirchner 1984; Armbruster 1985a, b; Putnis et al. 1985; Putnis et al. 1987; Malcherek et al. 2001; Bertoldi et al. 2004 and references therein; Balassone et al. 2004; Miletich et al. 2010). The tetrahedral positions are occupied by Si and Al. Cordierite occurs in two polymorphs which are distinguished by the Al/Si ordering. Most of the natural cordierites adopt the structure of the orthorhombic low-temperature polymorph with space group symmetry Cccm and a fully ordered Al/Si distribution where Al occupies the T11 and T26 positions and Si occupies the T16, T21 and T23 tetrahedral sites (e.g., Cohen et al. 1977; Meagher and Gibbs 1977; Hochella et al. 1979). The hexagonal high-temperature polymorph indialite (Miyashiro 1957) with space group symmetry P6/mcc shows a disordered Al/Si distribution and is isostructural with beryl (e.g., Meagher and Gibbs 1977 and references therein). The T1 tetrahedra are preferentially occupied by Al and the T2 tetrahedra by Si (e.g., Meagher and Gibbs 1977; Putnis et al. 1985; Putnis and Angel 1985; Winkler et al. 1991). Selected bond distances and angles of the Mg-cordierite polymorphs are given in Table 1. The information provided for hexagonal Mg-cordierite is based on crystallographic data of Winkler et al. (1991). The octahedral M-sites are mainly occupied by Mg and Fe2+ and Mg-Fe mixing in orthorhombic cordierite is assumed to be ideal (e.g., Boberski and Schreyer 1990). 90 to 99 % of the total Fe2+ occupies the octahedral site (Khomenko et al. 2001). The amount of Fe3+ in natural cordierites is insignificant (Geiger et al. 2000). The stacked tetrahedral rings form channels parallel to the crystallographic c-axis which can incorporate molecules such as CO2 and H2O, and also large cations such as Na+ or K+. Two channel sites (Ch0, Ch¼) can be distinguished in the structure, according to the general formula: (M)2(T11)2(T26)2(T23)2(T21)2(T16)O18(Ch0,Ch1/4). Most natural cordierites show XFe (Fe/Fe + Mg + Mn) <0.9. Under certain experimental conditions it was possible to synthesize pure Fe-cordierites (e.g., Gunter et al. 1984; Boberski and Schreyer 1990; Kaindl et al. 2011). The incorporation of Fe2+ into the octahedral positions of orthorhombic cordierite then leads to multiple structural changes when compared with Mg-cordierite. The average M-O distance increases which leads to an elongation of the crystallographic a and b-axis. The average T11-O distances decrease slightly resulting in a contraction of the c-axis (e.g., Wallace and Wenk 1980; Armbruster 1985b; Boberski and Schreyer 1990; Malcherek et al. 2001; Geiger and Grams 2003). All polyhedra in both cordierite polymorphs are distorted. The polyhedral distortion can be quantified with the quadratic elongation λ and the angle variance σ2 (Robinson et al. 1971) and these parameters are also given in Table 1. Several authors investigated cordierite with spectroscopic methods focussing on topics like polymorphism and Al/Si ordering (Langer and Schreyer 1969; McMillan et al. 1984; Güttler et al. 1989; Poon et al. 1990), volatile content (Armbruster and Bloss 1982; Vry et al. 1990; Kalt 2000; Kolesov and Geiger 2000; Yakubovich et al. 2004; Bul’bak and Shvedenkov 2005; Khomenko and Langer 2005; Kaindl et al. 2006; Kolesov 2006; Nasdala et al. 2006; Rigby et al. 2008; Weikusat et al. 2010), structural heterogeneity as well as energetic and structural changes under high pressure (Geiger and Grams 2003; Likhacheva et al. 2011). Group theoretic analysis yields 36 Raman active vibrations for hexagonal Mg-cordierite and 87 for the orthorhombic end-member (e.g. McMillan et al. 1984; Poon et al. 1990; Kaindl et al. 2011). Local structural heterogeneities and thermodynamic mixing properties in natural Fe-Mg-cordierites have also been studied with IR spectroscopy (Geiger and Grams 2003). The synthetic Fe-end-member has been investigated with X-ray powder diffraction and IR spectroscopic methods (Gunter et al. 1984; Boberski and Schreyer 1990) but not using Raman spectroscopy so far. Quantum-mechanical calculations of Raman spectra of ordered Mg- and Fe-cordierites yielded a detailed peak assignment and a theoretical description of the effects of the Mg-Fe exchange and Al/Si ordering (Kaindl et al. 2011). The effect of Fe incorporation on six selected Raman bands of synthetic hydrous orthorhombic cordierite has been investigated by Haefeker et al. (2012). The aim of this work is to provide detailed structural data for both polymorphs of synthetic Fe-cordierite and for the first time Raman spectroscopic data for the hexagonal polymorph. A thorough literature search showed that appropriate crystallographic data of both polymorphs and Raman spectra of synthetic hexagonal Fe-cordierite are not available in the literature yet. The investigations described in this contribution include comprehensive structural refinements based on X-ray single-crystal and powder diffraction, as well as descriptions of single bands and peaks of hexagonal cordierite Raman spectra. A method based on the comparison of tetrahedral volumes was applied to obtain information about the Al/Si ordering in hexagonal Fe-cordierite.
Experimental methods
Synthesis of Fe-cordierite
Disordered hexagonal and ordered orthorhombic Fe-cordierites were synthesized from stoichiometric glass using annealing and hydrothermal techniques. The detailed description can be found in Kaindl et al. (2011).
An oxide mixture with the stoichiometry Fe2.3Al4Si5O18 was molten in reducing atmosphere and then quenched in distilled water. Apart from elementary iron, which could be found as sparsely scattered small spheres, the glass was homogenous and showed the composition Fe2Al4Si5O18. Tempering the glass at 900 °C for 21 h together with graphite-powder in a sealed gold capsule lead to the crystallization of disordered hexagonal cordierite. A higher degree of Si-Al-ordering in the orthorhombic end-product was achieved by additional hydrothermal treatment for 2 weeks at 700 °C and 2 kbar.
Raman spectroscopy
Raman spectroscopic investigations were performed using a Labram HR-800 confocal Raman-spectrometer by HORIBA under ambient conditions and in a Linkam THMS 600 cooling stage at −190 °C. A 100x objective with a numerical aperture of 0.9 was used for the measurements under ambient conditions and a 50x objective with a numerical aperture of 0.5 was used in combination with the cooling stage. Unpolarized spectra of randomly oriented hexagonal Fe-cordierite crystal aggregates were recorded using the 532 nm excitation wavelength of a 30 mW Nd-YAG laser as well as the 633 nm line of a 17 mW He-Ne-laser. Small crystal sizes and/or twinning hampered single-crystal measurements. The spectra were recorded in the range of 100–1,250 cm−1. During all measurements the confocal pinhole aperture was 1,000 μm and the width of the entrance slit was 100 μm. A grating with 1,800 lines/mm was used to disperse the scattered Raman light and an open-electrode charge-coupled device with 1,024 × 256 pixels, each with a size of 43 μm, was used for detection. The spectral resolution of 1.4 cm−1 (green) and 0.9 cm−1 (red) was determined by measuring the Rayleigh line. To obtain high accuracy, the system was calibrated with emission lines of a Ne spectral calibration lamp and the resulting deviation was ±0.24 cm−1. The spectral software suite LabSPEC 5 (Horiba 2005) was used to fit the Raman spectra and the line-segments baseline correction and convoluted Gauss-Lorentz functions were used for peak fitting.
X-ray powder diffraction
A Bruker D8 Discover system with a Bragg-Brentano-geometry was used for powder X-ray investigations of the orthorhombic Fe-cordierite. The system was equipped with a silicon-strip-detector and a primary beam Qz(101)-monochromator. The sample was scanned over a 2Θ range from 5° to 80.25° in steps of 0.009°. The Mg-cordierite sample was investigated with a Stoe STADI-MP diffractometer system in bisecting transmission geometry. The system was equipped with a “Mythen” 1 k detector and an asymmetric primary beam Ge(111)-monochromator. Data were collected in a 2Θ range from 2 to 129.8° in steps of 0.009°. For LeBail fits and Rietveld calculations the program FullProf.2 k (Rodriguez-Carvajal 2011) was used.
Vestiges of hexagonal intermediates in the powder sample significantly complicated Rietveld refinements of orthorhombic Fe-cordierite data. LeBail fits (with R p = 1.94 %, R wp = 2.69 %, χ 2 = 3.24) yielded the lattice parameters given in Table 1 and the distortion index (Miyashiro 1957) of Δ ≈ 0.24. Rietveld refinements of the orthorhombic Mg-cordierite data (with R b = 3.47 %, R f = 2.79 %, R p = 4.65 %, R wp = 6.10 %, χ 2 = 2.58) yielded the structural information given in Table 1 with a distortion index Δ ≈ 0.24. The background was determined by linear interpolation between a set of breakpoints with refineable heights. Intensities within 10-times the full-width at half-maximum of a peak were considered to contribute to the central reflection. Thompson–Cox–Hastings pseudo-Voigt functions were chosen for the simulation of the peak shape, including an asymmetry correction following Finger et al. (1994).
Single-crystal X-ray diffraction
The disordered hexagonal and the ordered orthorhombic Fe-cordierite samples were measured at ambient conditions with a Stoe IPDS II single-crystal diffractometer using graphite monochromatized Mo-Kα radiation. After integration, data reduction included Lorentz and polarization corrections. Furthermore, an analytical absorption correction based on a description of the morphology by accurately indexed and measured external faces was applied. The structures were solved using the program Superflip (Palatinus and Chapuis 2007) and subsequently refined using Jana 2006 (Petricek et al. 2006). Neutral atom scattering coefficients and anomalous dispersion corrections were taken from the International Tables for Crystallography, Volume C (Wilson 1995). The parameters for X-ray data collection and structure refinement are given in Table 2. Orthorhombic Fe-cordierite shows pseudo-hexagonal micro-scale twinning and twinnig in synthetic orthorhombic Mg-cordierite has been described in literature (e.g., Putnis 1980; Armbruster 1985a; Blackburn and Salje 1999). The refinement of the orthorhombic Fe-cordierite was performed assuming three twin-domains, related to each other by a 60° rotation around c. One of the three twin individuals dominates the diffraction pattern with a volume fraction of 47 %. As a result, the symmetry of the diffraction pattern is clearly reduced to orthorhombic and up to the largest diffracting angle of 29.3° (Θ), no obvious splitting of the reflections could be observed. Using averaged reflection positions of the three pseudo-hexagonal twin domains for refinement of the lattice parameters forces the resulting cell to be of almost perfect hexagonal metric (a = 17.1538(11) b = 9.8689(6)): arctan(a/b) = 60.09°. The corresponding angle is 60.31° if the cell parameters determined from powder data are used. Unless the reflections of the twin-related domains can be resolved, ‘single-crystal lattice parameters’ will underestimate the orthorhombic distortion of the unit cell. As a consequence, we decided to use ‘powder lattice parameters’ (see section X-ray powder diffraction) for final geometry calculations. Residual electron density determined by difference Fourier-maps is related to H2O/Na+ contents in the channels. The VESTA 3 software (Momma and Izumi 2011) was used to visualize crystal structures and structural details as bond length, angles, polyhedral volumes and distortion parameters.
Electron microprobe analysis
A JEOL JXA 8100 SUPERPROBE at the Institute of Mineralogy and Petrography at the University of Innsbruck was used for chemical analysis of the synthesis products. The Fe-cordierite single-crystal used for structural investigations was embedded randomly oriented in an epoxy mount, ground and finally polished using a 1 μm diamond suspension. Analytical conditions for the wavelength dispersive measurements were 15 kV acceleration voltage, a beam current of 10 nA, and counting times of 20 s for the peak and 10 s for the background. Calibration was done using the following microprobe standards (standardized element in parenthesis): jadeite (Na), corundum (Al), almandine (Fe) and orthoclase (Si). The analysis yielded the following composition (wt.-%, the numbers in parentheses are 2σ standard deviations): FeO = 21.12(30), SiO2 = 44.72(32), Al2O3 = 31.06(26), Na2O = 0.16(4), ∑ 97.06. The average mineral formula was then calculated on the anhydrous basis of 18 oxygens, to be Fe1.96Al4.064Si4.963O18, Na0.035. An exact quantification of the channel-H2O is missing and the estimated amount is 1.5–2wt.-%.
Results
Crystal structure and Raman spectra of Fe2+-cordierites
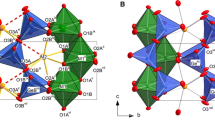
The atomic coordinates and equiv. displacement parameters of hexagonal Fe-cordierite are presented in Table 3. Anisotropic displacement parameters can be found in the attached cif-files that have been deposited as Supplementary material. Information about the unit cell (lattice parameters, volume) and single polyhedra (average distances, volumes, distortion parameters) is given in Table 1. Selected distances and angles (T-O, M-O, O-O) are presented in Table 4. The crystal structure is shown in Fig. 1.
The Raman spectra of synthetic hexagonal Fe-cordierite at ambient conditions and at −190 °C are shown in Figs. 2a-c and the Raman band positions taken from Fig. 2a (532 nm) are given in Table 5. The most intensive peak occurs at 568 cm−1. Characteristic for the low-frequency region of the spectrum are a low-intensity peak at 372 cm−1 and sharp low- to medium-intensity peaks at 111 and 257 cm−1. The latter overlaps with low-intensity peaks at 238, 272 and 297 cm−1. A broad band system contains three low-intensity peaks at 437, 458 and 482 cm−1. The high-frequency region is characterised by low-intensity peaks at 661 and 724 cm−1. Two low-intensity band systems containing overlapping broad peaks occur at 1) 908, 968, 1,019 and 1,051 cm−1 and 2) 1,105, 1,139 and 1,177 cm−1. Variations of peak intensities in Fig. 2a (532 nm) and Fig. 2b (633 nm) are the result of crystal orientation.
The atomic coordinates and equivalent displacement parameters of orthorhombic Fe-cordierite are listed in Table 6. Information about the unit cell (lattice parameters, volume) and polyhedra (average distances, volumes, distortion parameters) is given in Table 1. Selected distances and angles (T-O, M-O, O-O) are summarized in Table 7. The crystal structure is shown in Fig. 3.
Al, Si distribution
Calculations based on the comparison between the tetrahedral volumes in ordered and disordered cordierites can be used to estimate the Al/Si distribution in hexagonal cordierite. The comparison of the five tetrahedral volumes of the fully-ordered naturally occurring Mg-rich Whitewell-cordierite (Cohen et al. 1977) and a highly ordered pure synthetic Mg-cordierite (Kaindl et al. 2011; Haefeker et al. 2012) show only small deviations of 0.8–2.2 %. Based upon site occupancies and their position within the cordierite’s framework five individual tetrahedral volumes in the ordered orthorhombic (T11, T16, T21, T23, T26) and two in the disordered hexagonal cordierites (T1,T2) can be distinguished. In orthorhombic Fe-cordierite (Table 1), the volume of T16(Si) with 2.179 Å3 represents 0 % Al occupation and the volume of T11(Al) with 2.590 Å3 represents 100 % Al occupation, leading to the following linear equation to estimate the Al/Si distribution in the ‘mixed’ T1 tetrahedra:
In hexagonal Fe-cordierite the T1 volume of 2.481 Å3 (Table 1) is occupied by ~73 % Al. The equation for T2
is based on the average orthorhombic T21(Si)/T23(Si) volume of 2.167 Å3 and the T26(Al) volume of 2.671 Å3. The T2-volume of 2.308 Å3 in hexagonal Fe-cordierite (Table 1) corresponded to an occupancy of ~28 % Al. Expressed in atoms per formula unit (a.p.f.u.) this amounts to 2.2 Al and 0.8 Si a.p.f.u. for T1 and 1.7 Al and 4.3 Si a.p.f.u. for T2 (∑ = 3.9 Al and 5.1 Si).
For hexagonal Mg-cordierite (Table 1) the equations
and
were developed similarly thus yielding ~67 % Al in T1 and ~32 % Al in T2 tetrahedra, this amounts to 2.0 Al and 1.0 Si a.p.f.u. for T1 and 1.9 Al and 4.1 Si a.p.f.u. for T2 (∑ = 3.9 Al and 5.1 Si). Tetrahedral volumes were calculated based on the structural data of a synthetic orthorhombic Mg-cordierite from Haefeker et al. (2012) and data for hexagonal Mg-cordierite of Winkler et al. (1991).
Discussion
Determination of the octahedral diameter in Mg-Cordierite
The diameter of the octahedra in direction of the c-axis can be used as useful indicator to explain structural changes in cordierite and has to be determined differently for both polymorphs. The octahedra of the hexagonal Mg-cordierite show two parallel faces perpendicular to the c-axis with a face-center-face distance of 1.982 Å. In the orthorhombic polymorph these octahedra faces are tilted and the face-center-face distances have to be averaged to obtain comparable data. Both octahedral faces are defined by three oxygen atoms with different fractional z coordinates. Constructing (001) planes perpendicular to the c-axis, each passing through one of the related oxygens, yields the three different OPlane-center-OPlane distances of namely 2.049 Å, 2.047 Å and 1.872 Å. Their average leads to an octahedral diameter in direction of the c-axis of 1.989 Å. The octahedral diameters of Fe-cordierite are discussed below.
Hexagonal Fe-cordierite
Fe2+ incorporation into synthetic hexagonal cordierite leads to an elongation of the unit cell in a-direction and to a contraction in c-direction. In the Fe end-member the unit cell dimensions of a = 9.8801(6) Å and c = 9.2852(5) Å result in a unit cell volume of 784.95(8) Å3, which is ~1.7 % larger than the cell volume of synthetic Mg-cordierite. The larger Fe atom in the octahedrally coordinated M-sites leads to an increase of the M-O distances to 2.153(3), resulting in a ~5.5 % volume increase of the octahedra to 12.4 Å3. The T1-O distances decrease slightly to 1.714(2) Å and the average T2-O distance increases to 1.644(2) Å. The volumes of the T1 tetrahedra decrease to 2.481 Å3 and the volumes of the T2 tetrahedra increase to 2.308 Å3. The distortion parameters are given in Table 1. The O-Fe-O angles in the octahedra scatter extensively with values of 73.66(9)°, 84.58(8)° and 101.05(9)°. This leads to distinct variations in the O-O distances and thus the lengths of the edges of the octahedron. As shown in Fig. 4, the octahedron shows two faces perpendicular to the c-axis and angles of 101.05(8)° between the three octahedron’s axes. The octahedral diameter along c is 1.953 Å and thus 0.029 Å smaller when compared to the Mg polymorph. This is assumed as the main reason for the contraction of the c lattice parameter. The adjacent T2 tetrahedra show slightly increased (0.0034 Å) O-O distances of 2.690(3) Å parallel to c.
The Raman spectrum of the hexagonal Fe-cordierite at ambient conditions (Fig. 2a, b) shows peak shifts towards lower wavenumbers as a consequence of Fe incorporation when compared to hexagonal Mg-cordierite. Selected peak positions of hexagonal Mg-cordierite from McMillan et al. (1984) are given in parentheses below. In the Fe end-member the peaks at 111 cm−1 (120 cm−1) and 661 cm−1 (672 cm−1) show the highest down-shifts of 9 cm−1 and 11 cm−1 whereas the peak at 372 cm−1 (373 cm−1) shows only a small shift of 1 cm−1. The peaks at 257 cm−1 (256 cm−1) and 568 cm−1 (567 cm−1) show a slightly opposite shift direction of 1 cm−1. Structural similarities between the hexagonal and the orthorhombic cordierite allow peak assignments on the basis of the quantum-mechanical calculations of Kaindl et al. (2011). The peak at 111 cm−1 is mainly the result of bending motions of the T1 tetrahedra and the octahedra. Peaks between 230 and 257 cm−1 are mainly related to bending of tetrahedra and octahedra. The peak at 372 cm−1 is related to the bending vibrations of the octahedra and T2 tetrahedra. The region 400–500 cm−1 is dominated by bending vibrations of the tetrahedra. The intensive peak at 568 cm−1 is related to complex tetrahedral and octahedral stretching vibrations and the peak at 661 cm−1 is a result of the T1-bending vibrations. Bands in the range 900–1,200 cm−1 are due to stretching vibrations of the tetrahedra.
Figures 2b and c show the 100–1,250 cm−1 region of the spectrum of hexagonal Fe-cordierite measured at ambient conditions and at −190 °C with 633 nm laser excitation. Peak intensity deviations from Fig. 2a are the result of crystal orientation. The lower the temperature the slightly sharper the peaks become when compared to the spectrum measured at ambient conditions (Fig. 2b, c). The FWHMs of the peak at 257 cm−1 decrease from 17 to 13, at 370 cm−1 from 14 to 10 and at 567 cm−1 from 25 to 21. On the other hand the peak positions do not change significantly (<0.5 cm−1), indicating only a low thermal contribution (e.g. Hochella et al. 1979; Haussühl et al. 2011). In Hochella et al. (1979) the temperature-dependent expansion of the axes of orthorhombic cordierite with X Fe = 0.83 is given with 0.12–0.16 % when heated from 24 to 375 °C. The expansion of hexagonal Mg-bearing cordierite (indialite) in a and b is reported with <0.1 % at 200 °C but c decreases slightly. Poon et al. (1990) described the existence of a weak mode on the high-energy side of the 566 cm−1 peak in the spectrum of disordered Mg-cordierite at 253 °C. This peak could not be confirmed for the Fe-end-member in the low-temperature measurement.
Orthorhombic Fe2+-cordierite
Synthetic ordered Fe-cordierite has an orthorhombic unit cell with a = 17.2306(2), b = 9.8239(1) and c = 9.2892(1) Å and the volume increases as a consequence of Fe incorporation by 1.5 % to 1572.40(3) Å3 when compared with the Mg-end-member. The octahedral volume and distortion increase (Table 1), the O-M-O angles scatter extensively (Fig. 5, Table 7) with values of 70.70(18)°, 74.51(16)°, 84.56(19)°, 84.93(16)°, 98.40(17)°, 102.39(15)°, 102.81(17)°. The volume increase is related to an expansion in the a-b-plane only. A detailed representation of the octahedron is shown in Fig. 5. The average octahedral diameter in direction of the c-axis, which commensurates with the average distance of the shaded octahedral faces, is reduced to 1.948 Å (1.981 Å, 2.038 Å, 1.824 Å) and the corresponding angles show values of 102.39(15)°, 102.81(17)° and 98.40(17)°, which increase by 1–1.5° when compared to the Mg-end-member. The reduced octahedral diameter is the main reason for the decrease of the c lattice parameter as a function of Fe incorporation. Tetrahedral edges in direction of the c-axis show a higher O-O-distance variation as a consequence of the more distorted and tilted octahedra. In T23 and T26 this distance increases to 2.664(10) Å and 2.820(8) Å, in T21 it decreases to 2.607(8) Å thus yielding an average of 2.697(8) Å.
Conclusions
The octahedral expansion as a function of Fe incorporation occurs in both synthetic hexagonal as well as orthorhombic cordierite only in the a-b-plane. The tetrahedral and octahedral interplay results in a notable reduction of the octahedral diameters parallel to c, leading to complex changes of polyhedral distortions. The presented site occupancy determinations based on tetrahedral volumes indicate that the T1 tetrahedra in hexagonal Fe-cordierite are significantly enriched in Al and the T2 tetrahedra contain more Si. Since the O-O distances of the T2-tetrahedra parallel to c do not change significantly in the hexagonal and decrease only slightly in the orthorhombic polymorph, the overall reduction of the c axis of cordierite as a function of Fe incorporation is mainly due to changes within the octahedral layer.
From group theoretical analysis 36 Raman active vibrational modes are expected for hexagonal Fe-cordierite of which 21 can be experimentally observed. Although the quantum-mechanical calculations of Kaindl et al. (2011) were done for the orthorhombic polymorph, structural similarities allow the assignment of the observed Raman bands in the spectrum of the hexagonal polymorph to specific vibrational modes involving tetrahedral and octahedral sites. Similar calculations for the hexagonal polymorph are hampered by the Al/Si disorder.
References
Armbruster T (1985a) Crystal structure refinement, Si, Al-ordering, and twinning in “pseudo-hexagonal” Mg-cordierite. N Jb Mineral Mh 6:255–267
Armbruster T (1985b) Fe-rich cordierites from acid volcanic rocks, an optical and x-ray single-crystal structure study. Contrib Mineral Petrol 91:180–187
Armbruster T, Bloss FD (1981) Mg-Cordierite: Si/Al ordering, optical properties, and distortion. Contrib Mineral Petrol 77:332–336
Armbruster T, Bloss FD (1982) Orientation and effects of channels H2O and CO2 in cordierite. Am Mineral 67:284–291
Balassone G, Franco E, Mattia CA, Puliti R (2004) Indialite in xenolithic rocks from Somma-Vesuvius volcano (Southern Italy): crystal chemistry and petrogenetic features. Am Mineral 89:1–6
Bertoldi C, Proyer A, Garbe-Schönberg D, Behrens H, Dachs E (2004) Comprehensive chemical analyses of natural cordierites: implications for exchange mechanisms. Lithos 78:389–409
Blackburn JF, Salje EKH (1999) Time evolution of twin domains in cordierite: a computer simulation study. Phys Chem Miner 26:275–296
Boberski C, Schreyer W (1990) Synthesis and water contents of Fe2+-bearing cordierites. Eur J Mineral 2:565–584
Bul’bak TA, Shvedenkov GY (2005) Experimental study on incorporation of C-H-O-N fluid components in Mg-cordierite. Eur J Mineral 17:829–838
Cohen JP, Ross FK, Gibbs GV (1977) An X-ray and neutron diffraction study of hydrous low cordierite. Am Mineral 62:67–78
Finger LW, Cox DE, Jephcoat AP (1994) A correction for powder diffraction peak asymmetry due to axial divergence. J Appl Cryst 27:892–900
Geiger CA, Grams M (2003) Cordierite IV: structural heterogeneity and energetics of Mg–Fe solid solutions. Contrib Mineral Petrol 145:752–764
Geiger CA, Rager H, Czank M (2000) Cordierite III: the site occupation and concentration of Fe3+. Contrib Mineral Petrol 140:344–352
Gibbs GV (1966) The polymorphism in cordierite I: the crystal structure of low cordierite. Am Mineral 51:1068–1087
Gunter AE, Skippen GB, Chao GY (1984) Cell dimensions, Mössbauer and infrared-absorption spectra of synthetic cordierite. Can Mineral 22:447–452
Güttler B, Salje E, Putnis A (1989) Structural states of Mg cordierite III: infrared spectroscopy and the nature of the hexagonal-modulated transition. Phys Chem Miner 16:365–373
Haefeker U, Kaindl R, Tropper P (2012) Semi-quantitative determination of the Fe/Mg ratio in synthetic cordierite using Raman spectroscopy. Am Mineral 97:1662–1669
Haussühl E, Vinograd VL, Krenzel TF, Schreuer J, Wilson DJ, Ottinger J (2011) High temperature elastic properties of Mg-cordierite: experimental studies and atomistic simulations. Z Kristallogr 226:236–253
Hochella MF, Brown GE, Ross FK, Gibbs GV (1979) High-temperature crystal chemistry of hydrous Mg- and Fe-cordierites. Am Mineral 64:337–351
Horiba (2005) LabSPEC spectral software suite (Version 5.19.17). HORIBA Jobin Yvon, Villeneuve d’Ascq, France
Kaindl R, Tropper P, Deibl I (2006) A semi-quantitative technique for determination of CO2 in cordierite by Raman spectroscopy in thin sections. Eur J Mineral 18:331–335
Kaindl R, Többens D, Haefeker U (2011) Quantum-mechanical calculations of the Raman spectra of Mg- and Fe-cordierite. Am Mineral 96:1568–1574
Kalt A (2000) Cordierite channel volatiles as evidence for dehydration melting: an example from high-temperature metapelites of the Bayerische Wald (Variscan belt, Germany). Eur J Mineral 12:987–998
Khomenko VM, Langer K (2005) Carbon oxides in cordierite channels: determination of CO2 isotopic species and CO by single crystal IR spectroscopy. Am Mineral 90:1913–1917
Khomenko VM, Langer K, Geiger C (2001) Structural locations of the iron ions in cordierite. Contrib Mineral Petrol 141:381–396
Kolesov BA (2006) Raman spectra of single H2O molecules isolated in cavities of crystals. J Struct Chem 47:21–34
Kolesov BA, Geiger CA (2000) Cordierite II: the role of CO2 and H2O. Am Mineral 85:1265–1274
Langer K, Schreyer W (1969) Infrared and powder X-ray diffraction studies on the polymorphism of cordierite, Mg2(Al4Si5O18). Am Mineral 54:1442–1459
Likhacheva AY, Goryainov SV, Krylov AS, Bul’bak TA, Prasad SR (2011) Raman spectroscopy of natural cordierite at high water pressure up to 5 GPa. J Raman Spectrosc. doi:10.1002/jrs.3060
Malcherek T, Domeneghetti MC, Tazzoli V, Ottolini L, McCammon C, Carpenter MA (2001) Structural properties of ferromagnesian cordierites. Am Mineral 86:66–79
McMillan P, Putnis A, Carpenter MA (1984) A Raman-spectroscopic study of Al-Si ordering in synthetic magnesium cordierite. Phys Chem Miner 10:256–260
Meagher EP, Gibbs GV (1977) The polymorphism of cordierite II: the crystal structure of indialithe. Can Mineral 15:43–49
Miletich R, Gatta GD, Redhammer GJ, Burchard M, Meyer HP, Weikusat C, Rotiroti N, Glasmacher UA, Trautmann C, Neumann R (2010) Structure alterations in microporous (Mg, Fe)2Al4Si5O18 crystals induced by energetic heavy-ion irradiation. J Solid State Chem 183:2372–2381
Mirwald P, Kirchner A (1984) Zum Ordnungs-/Unordnungsverhalten von Mg-Cordierit zwischen 1400 °C und dem Schmelzpunkt (1463 °C). Fortschr Mineral 62:157–159
Miyashiro A (1957) Cordierite-indialite relations. Am J Sci 255:43–62
Momma K, Izumi F (2011) VESTA 3 for three-dimensional visualization of crystal, volumetric and morphology data. J Appl Cryst 44:1272–1276
Nasdala L, Wildner M, Wirth R, Groschopf N, Pal DC, Möller A (2006) Alpha particle haloes in chlorite and cordierite. Mineral Petrol 86:1–27
Palatinus L, Chapuis G (2007) Superflip - a computer program for the solution of crystal structures by charge flipping in arbitrary dimensions. J Appl Cryst 40:786–790
Petricek V, Dusek M, Palatinus L (2006) Jana 2006. The crystallographic computing system. Institute of Physics, Praha, Czech Republic
Poon WCK, Putnis A, Salje E (1990) Structural states of Mg cordierite: IV. Raman spectroscopy and local order parameter behaviour. J Phys Condens Matter 2:6361–6372
Putnis A (1980) Order-modulated structures and the thermodynamics of cordierite reactions. Nature 287:128–131
Putnis A, Angel RJ (1985) Al, Si ordering in cordierite using “magic angle spinning” NMR. II: models of Al, Si order from NMR data. Phys Chem Miner 12:217–222
Putnis A, Bish DL (1983) The mechanism and kinetics of Al, Si ordering in Mg-cordierite. Am Mineral 68:60–65
Putnis A, Fyfe CA, Gobbi GC (1985) Al, Si ordering in cordierite using “magic angle spinning” NMR. I. Si29 spectra of synthetic cordierites. Phys Chem Miner 12:211–216
Putnis A, Salje E, Redfern SAT, Fyfe CA, Strobl H (1987) Structural states of Mg-cordierite I: order parameters from synchrotron X-ray and NMR data. Phys Chem Miner 14:446–454
Rigby MJ, Droop GTR, Bromiley GD (2008) Variations in fluid activity across the Etive thermal aureole, Scotland: evidence from cordierite volatile contents. J Metamorph Geol 26:331–346
Robinson K, Gibbs GV, Ribbe PH (1971) Quadratic elongation: a quantitative measure of distortion in coordination polyhedra. Science 172:467–570
Rodriguez-Carvajal J (2011) FullProf.2k, Version 5.20, Institut Laue-Langevin, Grenoble, France
Vry JK, Brown PE, Valley JW (1990) Cordierite volatile content and the role of CO2 in high-grade metamorphism. Am Mineral 75:71–88
Wallace JH, Wenk HR (1980) Structure variation in low cordierites. Am Mineral 65:96–111
Weikusat C, Miletich R, Glasmacher UA, Trautmann C, Neumann R (2010) Heavy-ion irradiation on crystallographically oriented cordierite and the conversion of molecular CO2 to CO: a Raman spectroscopic study. Phys Chem Miner 37:417–424
Wilson AJC (1995) International tables for crystallography, Vol. C: mathematical, physical and chemical tables. Kluwer Academic Publishers, Dordrecht, Boston, London
Winkler B, Dove MT, Leslie M (1991) Static lattice energy minimization and lattice dynamics calculations on aluminosilicate minerals. Am Mineral 76:313–331
Yakubovich OV, Massa V, Pekov IV, Gavrilenko PG, Chukanov NV (2004) Crystal structure of the Na-, Ca-, Be-cordierite and crystallochemical regularities in the cordierite-sekaninaite series. Crystallogr Rep 49:953–963
Acknowledgments
We thank Martina Tribus for her help with the electron microprobe analysis, Jürgen Konzett for his help with the crystal synthesis and Daniel Többens for his help with X-ray powder analysis. Financial support of the Austrian Science Fund (FWF): [P22013-N21 to R.K.] is gratefully acknowledged.
Author information
Authors and Affiliations
Corresponding author
Additional information
Editorial handling: A. Beran
Rights and permissions
Open Access This article is distributed under the terms of the Creative Commons Attribution License which permits any use, distribution, and reproduction in any medium, provided the original author(s) and the source are credited.
About this article
Cite this article
Haefeker, U., Kaindl, R., Tropper, P. et al. Structural investigations of the two polymorphs of synthetic Fe-cordierite and Raman spectroscopy of hexagonal Fe-cordierite. Miner Petrol 108, 469–478 (2014). https://doi.org/10.1007/s00710-013-0313-3
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00710-013-0313-3