Abstract
In this work, the involvement of programmed cell death (PCD) in the wound-induced postharvest browning disorder and senescence in butterhead lettuce (Lactuca sativa L.) fresh-cuts was studied. At the wounded (cut, bruised) sites, rapid browning, loss of chlorophyll and massive cell death, accompanied with accumulation of reactive oxygen species and increased electrolyte leakage occurred in a narrow strip of tissue adjacent the injury. The dead cell morphology (protoplast and nuclei shrinkage) together with the biochemical and physiological changes resembled necrotic PCD type. With a slight delay post-wounding, senescence associated with similar cell death features was initiated in distant non-wounded sites. In addition to necrotic PCD, both in wounded and senescing tissue, the appearance of empty cell corpses was observed, indicating that part of the cells might undergo vacuolar PCD (self-digestion of cellular content after vacuole collapse). The wounding-induced local cell death at the primary site of damage suggested that PCD may serve as a mechanism to seal-off the wound by building a physical barrier of dead cells. However, the cell death at sites remote from the wound suggests the distribution of long-distance senescence-inducing wound messengers. Trichomes in unwounded tissue often were the first to show H2O2 accumulation and dead cells; thereafter, the elevated H2O2 and cell death appeared in connecting cells and senescence progressed over larger areas. This suggests that trichomes may contribute to mediating the wound signalling leading to subsequent senescence. Our findings demonstrate that PCD is an integral part of the wound syndrome in fresh-cut lettuce.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
The shelf life of fresh-cut lettuce (a demanded ‘ready to use’ vegetable product) is largely dependent on factors such as genetic background, developmental stage at harvest of the starting material and postharvest handling conditions (Bolin et al. 1997; Gil et al. 2012; Martínez-Sánchez et al. 2012; Witkowska and Woltering 2013, 2014; Pareek 2016). During processing, the fresh-cuts suffer from wound stress resulting from cutting, bruising, folding, pressing and other mechanical interventions that disrupt the integrity and physiological functioning of the leaf tissues. Major deterioration in the leafy fresh-cuts is pinking and browning at the wounded sites (Couture et al. 1993; Castañer et al. 1996; Cantwell and Suslow 2002; Hodges and Toivonen 2008; Pedreschi and Lurie 2015). Among others, treatments with gaseous compounds (e.g. nitric oxide (NO), ozone, hydrogen sulphide), soluble substances with antioxidant properties, chlorine and calcium-based solutions, hot water, UV radiation, high pressure, modulations of light quality and photoperiod and, genetic manipulations are shown to suppress the wound-induced browning, delay senescence, stimulate the expression of defence genes or downregulate stress- and senescence-associated genes (Coupe et al. 2003; Rico et al. 2006; Eason et al. 2014; Li et al. 2014; Mahajan et al. 2014; Iakimova and Woltering 2015; Woltering and Seifu 2015). Storage under modified (MA) or controlled atmosphere (CA) with low O2 (< 3%) and increased CO2 levels (up to 10–15%) is another technology for preventing the occurrence of browning syndrome and premature senescence. (Ballantyne et al. 1988; López-Gálvez et al. 1996a; Fonseca et al. 2002).
Although the physiological, biochemical and molecular processes involved in browning and senescence disorders have gotten appropriate attention (e.g. Hodges and Toivonen 2008; Pareek 2016), still little is known about the cellular changes underlying the wound response in fresh-cuts and particularly at the primary site of injury. Wound-induced browning is generally attributed to the production of phenolic compounds linked to the activity of polyphenol oxidase, phenylalanine ammonia lyase and peroxidase and is defined as enzymatic browning (Couture et al. 1993; Pereyra et al. 2005; López-Gálvez et al. 1996b; Degl'Innocenti et al. 2007; Saltveit and Choi 2007). Recent works suggested that lysophospholipids are the most probable primary wound signals involved in the formation of browning substances (García et al. 2017; Saltveit 2018).
An advanced view is that postharvest deterioration of fresh vegetables and fruits might be related to the occurrence of programmed cell death (PCD). It is observed that storage-induced disorders such as chilling injuries and low O2 and high CO2 disorders are often accompanied by death and sometimes disappearance of cells at specific locations. Fluids from dying cells may leak into the intercellular spaces causing macroscopic signs of deterioration (e.g. brown, sunken or water soaked lesions, scald and tissue dismantlement) (Cantwell and Suslow 2002; Coupe et al. 2003; Fernández-Trujillo and Martínez 2006; Saltveit and Choi 2007; Hurr et al. 2010; Woltering and Iakimova 2010; Eason et al. 2014; Iakimova and Woltering 2015; Cantre et al. 2017). The understanding of the role of PCD in postharvest disorders is, however, still in its infancy.
PCD is a highly coordinated process of cellular suicide. In eukaryotic systems, it is a part of the normal development and can operate as a survival mechanism at stressful circumstances (Pennell and Lamb 1997; Gunawardena et al. 2001; Lam 2004; Reape et al. 2008). According to the morphological classification introduced by van Doorn et al. (2011), plant PCD is defined in two major categories: vacuolar cell death and necrosis. Vacuolar cell death is featured by autophagic activity such as formation of lysosome-like lytic organelles, vacuolar growth and activation of vacuolar processing enzyme (VPE), tonoplast rupture and vacuole-mediated digestion of the cellular content leaving a virtually empty cell corpse behind (van Doorn and Woltering 2010). Hallmarks of necrotic cell death are swelling of mitochondria and changes of membrane permeability, early rupture of plasma membrane and electrolyte leakage, protoplast shrinkage and nucleus compaction. Necrotic PCD is associated also with respiratory decline, ATP depletion, diminished photosynthetic activity and oxidative stress-related processes. This type of cell death results in a largely unprocessed cell corpse. DNA degradation yielding a ladder pattern, due to enzymatic cleavage of DNA into oligonucleosomal fragments of 180 bp and multiples thereof, and activation of cell death related plant caspase-like proteases that are functional homologues of caspases (cysteinyl-aspartic proteases—the main executioners of animal apoptotic PCD) (Woltering 2010) may occur in both plant PCD categories. Forms of PCD expressing mixed phenotype are classified as cell death modalities. An example is the hypersensitive response (HR)—rapid local cell death occurring in plant-microbe interactions and aimed at suppressing the pathogen growth and restricting the infection to the primary site of microbial attack (Levine et al. 1994; Mur et al. 2008). Senescence and most cases of developmental PCD (e.g. xylogenesis) are generally thought to conform to the vacuolar cell death type. However, in senescing leaves and petals and in differentiating xylem vessels distinct and similar molecular patterns and physiological processes reminiscent of necrotic cell death have also been documented (Quirino et al. 2000; van Doorn and Woltering 2004, 2005, 2008; Lim et al. 2007; Price et al. 2008; Shibuya et al. 2016; Iakimova and Woltering 2017). We support the concept that the entire process of senescence is a PCD event in which autophagy in the early phases and the final culmination of cellular demise are tightly integrated. PCD—related gene expression, signalling pathways and the autophagic activity are initiated and cells acquire competence for undergoing cell death early in advance of the cell death execution phase (Yen and Yang 1998; Quirino et al. 2000; van Doorn and Woltering 2004; Shibuya et al. 2016; In this paper senescence is considered in this context.
The occurrence of PCD in senescing postharvest lettuce has been so far a subject of only few works. Wagstaff et al. (2007) found that the reduced shelf life of harvested baby lettuce leaves was associated with disruption of plastid membranes and the nuclear envelope, plasmolysis and electrolyte leakage. Electron microscopy disclosed the presence of cytoplasmic fragments in the vacuole and increased appearance of vesicles and microbodies in mesophyll and epidermal cells. The authors also observed disappearance of cells in the leaf tissue. These features resemble mainly the class of vacuolar cell death with some features of necrotic PCD. Features reminiscent of vacuolar PCD were also described in fresh-cuts of asparagus lettuce which were subjected to high pressure (above 100 MPa) processing. The excessive pressure caused cell death characterised by formation of vesicles in the cytoplasm, disappearance of chloroplasts and vacuole rupture (Zhang et al. 2015). Investigations on broccoli florets showed that quality decline during postharvest period was accompanied by cell death expressing common hallmarks of necrotic and vacuolar PCD such as DNA laddering and increasing number of TUNEL (terminal deoxynucleotidyl transferase-mediated dUTP nick end labelling) positive nuclei (a marker of double-strand DNA brakes). Tissue deterioration involved also changes in expression of the PCD-related genes LSD1 (lesion simulating disease), Bax inhibitor (BI) and serine palmitoyltransferase (SPT), an enzyme in the sphingolipid signalling pathway (Coupe et al. 2004). Downregulation of cell death, senescence and stress-associated genes encoding for cysteine proteases such as BoCP1, BoCP2, BoCP3 and BoCP4 was documented to reduce the dehydration and delay senescence of postharvest broccoli (Coupe et al. 2003, 2004; Gapper et al. 2005; Eason et al. 2014). Bioengineering manipulation of the hormonal status is another approach for regulating the occurrence of cell death and senescence postharvest. For example, McCabe et al. (2001) reported that senescence in harvested mature heads of homozygous transgenic lettuce transformed with Arabidopsis ipt gene (encoding for isopentenyl phosphotransferase—enzyme from cytokinin biosynthesis and under control of the senescence-specific SAG12 promoter) was significantly retarded. Leaf senescence was also largely prevented in mutant lettuce and broccoli with suppressed ethylene synthesis genes (Henzi et al. 2000; Buchanan-Wollaston et al. 2003; Gapper et al. 2005).
Together, the mentioned findings indicate that in the leafy vegetables PCD processes may be responsible for at least some of the postharvest disorders. However, the cellular bases of wound-induced deterioration need to be better elucidated.
The rapid occurrence of browning in fresh-cut lettuce, especially at the primary site of wounding, makes this product an appropriate model to study the contribution of PCD to the wound response. In the present study wound-induced PCD events in lettuce (Lactuca sativa L.) fresh-cuts are addressed. Morphological, physiological and biochemical determinants of cell death were identified by applying a combined analytical approach involving microscopy, histochemical and quantitative image analyses, biochemical assay and visual observations. It is shown that PCD is an integral part of the browning and senescence syndrome in lettuce fresh-cuts. The process may serve for building a physical barrier for preventing the spread of cell death from the wounded site. The observations suggest that a wound signal generated at the primary site of injury may be communicated toward unwounded remote cells. A possible role of trichomes in mediating long-distance wound signalling leading to consecutive senescence/cell death is discussed.
Materials and methods
Plant material and wounding treatments
Greenhouse grown butterhead lettuce (Lactuca sativa L.), cv. Cosmopolia was harvested at commercial maturity (4-week-old heads), transported to the laboratory, plastic covered and stored for 12 h in a cold room (4 °C and 96% relative humidity). The outer leaves of the heads were discarded; the leaves from the second and third whorls (mature leaves) inward from discarded ones were detached and midribs and major veins removed. With a sharp stainless steel knife these leaves were cut into pieces of approximate size 8 × 2 cm. To assess the effect of wounding, in addition to the damage at the cutting edge, in some leaf pieces, extra wounding was done by removal of a small tissue disc (0.5 cm diameter) using a cork borer, and the tissue at sites distant from the cut edge and limited to an area of approximately 1–5 mm was bruised with the tip of plastic syringe (without a needle). The additionally injured fresh-cuts were determined as group 1, (‘bruised shreds’) and the fresh-cuts subjected only to wounding at the cut surface were group 2 (‘non-bruised shreds’).
Storage conditions
The samples were placed in plastic boxes, the bottom of which was lined with moist filter paper (Whatman grade No. 3) for preventing the desiccation and with a layer of plasticized wire mesh to avoid the contact of plant tissues with the moist paper. The boxes were covered with transparent plastic lids punctured at 16 points (approximately 1 mm diameter) to allow sufficient gas exchange with the environment and prevent accumulation of CO2, ethylene and other gasses released from the plant material. The samples were stored in a climate room, at 4 °C, in darkness. The experiments were undertaken with 3–4 boxes (replicates) for analyses at each time point. In total, 5 independent experiments were performed.
Visual evaluation of deterioration and shelf life
Wound-induced browning deterioration was visually estimated by severity of browning at the cut surface and at locally wounded tissue in the ‘bruised shreds’, according to a scale previously described by Iakimova and Woltering (2015): 5—none; 4—slight; 3—moderate; 2—severe; 1—extreme browning. Senescence and shelf life were scored in the ‘non-bruised’ samples by combining overall visual quality (OVQ) and appearance of browning using two increment scale (1–9) and intermediate levels, partially adopted from Kader et al. (1973): 9—no yellowing, leaf tissues in full turgor, excellent, essentially free of defects; 7—good, minor reduction of leaf turgor, not objectionable yellowing and other defects; 5—fair, slightly to moderately objectionable senescence appearing as reduced leaf turgor, lower limit of sale appeal; 3—poor, advanced senescence expressed as excessive loss of leaf turgor and severe yellowing, limit of saleability; 1—extremely poor, very advanced senescence associated with severe tissue yellowing, necrotic lesions, desiccation and decay, not usable. The shelf life was considered terminated at OVQ rank below 5 and browning rank below 3.
Microscopy
Microscopy was performed on leaf discs (0.5 cm diameter) isolated with a cork borer from wounded sites (cut edges and bruising sites) and from non-wounded tissue. Presented micrographs of the histological analyses are representative examples of about 75 observed microscopy fields (5 fields per micrograph) in commonly 15 micrographs collected at each time point in 3 independent experiments.
Histochemical detection and quantification of H2O2
Hydrogen peroxide was distinguished by 3,3′-Diaminobenzidine (DAB) staining following the protocol of Thordal-Christensen et al. (1997) and as described by Iakimova and Woltering (2015). The samples were observed and imaged under light microscope Leitz Aristoplan equipped with Nikon Digital camera DMX 1200. In the presence of H2O2 DAB is polymerised giving a visible brown stain with intensity corresponding to the amount of H2O2.
The amount of H2O2 was quantified by pixel intensity of the brown DAB deposits measured with computer application ImageJ (Image Processing and Analysis Application in Java, National Institute of Health (NIH), USA) as described in Iakimova and Woltering (2015). Pixel intensities of DAB images (in gray values, background subtracted) range from 0 to 255. Value 0 corresponds to the darkest colour and 255 to the lightest colour in the image. Higher intensity corresponds to lower H2O2 amount.
Histochemical detection of overall ROS
The production of overall ROS was analysed by using the fluorescent probe 2′,7′-dichlorofluorescein diacetate (DCF-DA), according to Sakamoto et al. (2005). This dye is non-fluorescent in reduced form and readily permeates the plasma membrane. Once in the cell, non-specific esterases cleave its acetate groups and the dye becomes membrane impermeable, trapped inside the cell. When oxidised by H2O2, hydroxyl, peroxyl and other free oxygen radical products, DCF-DA is converted to the green fluorescing form 2′,7′-dichlorofluorescin and ROS appeared in green. Overall ROS were visualised in leaf discs (0.5 cm diameter), collected as described above. The samples were washed with distilled water and incubated in presence of 10 μmol l−1 DCF-DA for 60 min at room temperature, in darkness. The fluorescence emitted from stained ROS was detected under Zeiss Axioskop fluorescent microscope equipped with filter combination excitation/emission wavelength 490/525 nm and with Nikon Digital camera DMX 1200 for imaging.
Quantification of the fluorescence emitted from chlorophyll
The change in chlorophyll was estimated by the red fluorescence emitted at wavelength 490/525 (excitation/emission) by using Zeiss Axioskop fluorescent microscope equipped with Nikon Digital camera DMX 1200. Chlorophyll amount was quantitatively expressed in pixel intensity by analysing the images using ImageJ. Pixel intensity (indicating the presence of chlorophyll) was measured similarly to the described for H2O2 quantification. However, opposite to the readings for H2O2, the higher pixel intensity corresponds to higher chlorophyll amount. Zero value of grey corresponds to the lower level of fluorescence and value 255 represents the highest level.
Cell death determination
Cell death was analysed by Evans Blue and propidium iodide (PI) staining.
Evans Blue staining of the dead cells (the dye is excluded from the living cells) was performed according to Keogh et al. (1980), with slight modifications as described in Iakimova and Woltering (2015). The dead cells were identified by the blue coloration of their content (Evans Blue positive cells). Observations and imaging were done by light microscope Leitz Aristoplan equipped with Nikon Digital camera DMX 1200.
The dead cells were also distinguished by labelling with the fluorophore PI which penetrates the damaged plasma membrane and the nucleus. This dye emits red fluorescence after binding to DNA by intercalating between the bases with little or no sequence preference and with a stoichiometry per 4–5 base pairs of DNA. The stained cells are defined as PI positive. Following the manufacturer instructions (Molecular Probes, Inc.), leaf discs were incubated in 500 nmol l−1 PI (in dH2O2) for 1–5 min and then rinsed with dH2O2. The observations were done under fluorescent Zeiss Axioskop microscope, excitation/emission filters 530/625 nm. Images were taken with Nikon Digital camera DMX 1200.
Electrolyte leakage assay
In addition to Evans Blue and PI stainings, cell death was estimated by electrolyte leakage (EL) which is a marker of the permeability of the cellular membranes. The EL was determined by measuring the electrical conductivity (EC) according to Song et al. (2012) and as earlier described by Iakimova and Woltering (2015), and expressed in percentage, calculated using the formula: EL (%) = EC1initial conductivity/EC2 total conductivity × 100 where EC1 and EC2 are in microsiemens (μS).
Data analysis and artworks
The data were subjected to Student’s t test, one-way analysis of variance (ANOVA) at probability level P ≤ 0.05 (IBM SPSS Statistics). Graphic artworks were done by using MS Office Excel; the images were combined by MS Office Power Point and sized by Windows 64 Bit Software IrfanView.
Chemicals
All chemicals (if not otherwise indicated) used for the assays were purchased from Sigma-Aldrich Chemie B.V., Zwijndrecht, The Netherlands.
Results
Shelf life and tissue deterioration
The OVQ and the occurrence of browning in the ‘non-bruised’ fresh-cuts were visually evaluated from day 1 to day 9 of storage (Figs. 1 and 2). Yellowing and slight browning near the cut edge and around the other injured sites initially appeared on day 2 and the severity was increasing until the end of shelf life (Figs. 1 a1, b–d and 2b). The first symptoms of senescence (slight yellowing and minor loss of leaf turgor) in ‘non-bruised shreds’ were noticed around day 3 and in the following days the fresh-cuts completely senesced (Fig. 2a). After about 7 days, the quality reached the limit of acceptability (levels of OVQ below 5 and browning score below 3) (Figs. 1b and 2a, b). On day 9, necrotic lesions consisting of entirely disintegrated tissue were observed (Fig. 1d). At some places inside the necrotic lesions and also in non-necrotic senescing areas, the cells disappeared and this was already noticed on day 7 (Fig. 1c, d). In ‘bruised shreds’, rapid browning was observed at the cut edges and at the bruised sites (Fig. 1a1 and 1b) and early cell death was detected within the initially browning areas in a narrow strip of tissue surrounding the wounds (Evans Blue positive blue coloured cells) (Fig. 1a3). A symptom of PCD occurring rapidly after the wounding was also the increased level of H2O2 on day 2 (Fig. 1a2). The first areas with senescing tissue in ‘bruised shreds’ appeared distant of the wounded sites approximately 1 day after the initiation of visible browning. Further, senescence in unwounded tissue of these shreds developed in a manner similar to that in the ‘non-bruised’ shreds. Until the end of shelf life, the browning in both groups of samples remained confined to a zone bordering the cut edge and in the vicinity to the local bruises (Fig. 1b–d). These data showed that the wounding accelerates senescence in unwounded tissue and that, although with increasing severity, the browning deterioration developed only in vicinity of wounded sites.
Senescence, wound-induced browning and cell death in lettuce fresh-cuts, stored at 4 °C a1 Slight browning at the cut edge (non-labelled tissue). a2 H2O2 production at the area showing initial browning, DAB staining, chlorophyll removed—the tissue appears in brown due to the presence of H2O2. a3 Cell death surrounding an injured site; following Evans Blue staining the dead cells appear in blue (a1–3), day 2. b Tissue browning in area adjacent a site injured with a cork borer, day 4. c Senescing shred on day 7; note sites in which cells have disappeared (arrows); inset shows area with vanished cells. d Senescing shred on day 9; visible is severe browning close to the cut edge, a large necrotic lesion of entirely necrotized tissue and disappearance of cells inside and in vicinity to it (inset, arrows). Scale bars = a2 500 μm, a3 50 μm, c (inset) 100 μm
Time course and severity of deterioration of lettuce fresh-cuts stored at 4 °C a Overall visual quality (OVQ). b Browning severity. Dashed lines indicate the lower limit of consumer acceptance. Presented values are means ± SEM (n–1) (n = 20); 4 replicates per time point of fresh-cut samples prepared from 5 lettuce heads in each of 5 independent experiments. Data indicated with same letters do not differ significantly from each other at P ≤ 0.05
Chlorophyll loss
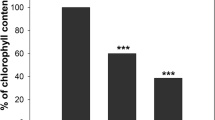
Red fluorescence of chlorophyll was visible by microscopy and quantified as a decrease in pixel intensity (Figs. 3 and 4). In freshly prepared fresh-cuts and after 1 day of storage, chlorophyll loss was not yet observed (Figs. 3a, e and 4). At later time points, the fluorescence gradually declined. A decrease of chlorophyll in wounded and senescing areas was very clear on day 4 of storage (Figs. 3b–d, f–h); chlorophyll more rapidly disappeared at the cut edge and bruised sites than in the non-wounded sites (Fig. 4). In the samples taken from brown tissue surrounding, the wounded sites (cut edge or bruises), on day 7 (end of shelf life, images not shown), the fluorescence was almost completely absent whereas in non-bruised senescing tissue it remained detectable albeit with lower intensity (Fig. 4). These observations showed that chlorophyll loss initially was restricted to a small area around the wounds, whereas senescence-associated chlorophyll breakdown in non-wounded senescing tissues occurred over the whole leaf area and was delayed in comparison to wounded sites.
Microscopy observations on chlorophyll loss in senescing ‘non-bruised’ and ‘bruised’ shreds of lettuce stored at 4 °C a Day 1 of storage. b Senescing area, day 4. c Cut edge, day 4. d Bruised site, day 4. e–h Fluorescence of chlorophyll in panels a–d, respectively. a–d Light microscopy; e–h Fluorescent microscopy. At sites loosing chlorophyll the red fluorescence is fading or not detectable. Scale bars = a and e 200 μm, b–d and f–h 100 μm
Fluorescence of chlorophyll at wounded and senescing sites of lettuce fresh-cuts stored at 4 °C Fluorescence is quantified by pixel intensity. Initial value (day 0) is shown as dotted line. Presented values are means ± SEM (n–1), (n = 25). Quantification was done in 5 non-overlapping microscopy fields in each of at least 5 representative micrographs collected from 3 independent experiments; each separate experiment was carried out with fresh-cut samples prepared from 5 lettuce heads. Data indicated with same letters do not differ significantly from each other at P ≤ 0.05
Production of H2O2 and overall ROS
The generation of ROS, including H2O2 was analysed histochemically by using specific labelling. DAB staining (formation of brown-coloured deposits) revealed massive H2O2 accumulation close to the cut edge. In bruised areas, H2O2 started to increase on day 2 (Fig. 1a2) and was well expressed after 4 days of storage (Fig. 5d, i). The diminution of pixel intensity (indicating H2O2 quantity) in the images supported this observation (Fig. 7). In comparison to day 0, no difference in H2O2 amount was detected on day 1. The level of H2O2 at the cut edge was increasing with advancement of tissue browning and cell death in vicinity of the wounds (Figs. 2b, 5b, d and 7). High levels of H2O2 were found also in xylem vessels and in their neighbouring cells within injured sites (Figs. 5d and 6h). These results pointed that enhanced H2O2 was confined to wounded areas.
Wound-induced cell death response in lettuce fresh-cuts stored at 4 °C a Evans Blue stained cut edge; the lack of blue coloured tissue indicates a lack of dead cells day 1. b Evans Blue stained dead cells at the cut edge; note the blue stained tissue, day 4. c PI stained dead cells in vicinity to cut edge, day 4; note the PI positive (red fluorescing) condensed nuclei. d H2O2 accumulation in vicinity to cut edge, day 4; DAB staining; note the brown coloured deposits. e Accumulation of overall ROS in vicinity to cut edge, day 4. DCF-DA staining; note the green fluorescence. f Evans Blue stained bruised leaf area; no blue labeled dead cells are detected, day 1. g Evans Blue stained bruised area; visible are dead cells with shrunken protoplast (in blue), day 4. h PI stained nuclei (bright red fluorescence) in dead cells in bruised area, day 4. i H2O2 accumulation in cells with shrunken protoplasts in bruised area; note the brown DAB deposits, day 4. j ROS accumulation (green fluorescing cloud) in bruised area, day 4; DCF-DA staining. a, b, d, f, g and i Light microscopy; c, e, h and j Fluorescent microscopy. dc Dead cell, n Nucleus, p Protoplast, s Stoma, v Vessel. Scale bars = a–e, i and j 100 μm, f–h 50 μm
Cell death in senescing lettuce fresh-cuts stored at 4 °C a Non-senescing area, day 1; on the right of the vessel—part of the fresh-cut with intact epidermis; on the left - the mesophyll layer with epidermis removed. b Living trichome, day 1. c Dead trichomes, day 4. a–c Chlorophyll removed. d Overall ROS (green fluorescence) in non-senescing area; the red fluorescence is emitted from chlorophyll in the living cells, day 1. e Overall ROS in senescing area, day 4. f H2O2 in non-senescing area; H2O2 is detectable by the brown DAB labelling inside the vessels, day 1. g H2O2 in dead trichome and in epidermal cells underneath; note the brown DAB deposits, day 2. h H2O2 in dead trichomes; xylem vessel heavily loaded with H2O2, day 4. i H2O2 in senescing area, day 4. j H2O2 in senescing area, day 7. i and j—note the spread and increasing intensity of the brown coloration. k Evans Blue stained non-senescing area; blue coloured dead cells are not detectable, day 1. l Dead cells in trichome; note the dead Evans Blue positive (blue) cells in the upper part and the living cells (Evans Blue negative) at the base of trichome, day 2. m Evans Blue stained dead cells in several dead trichomes and in the connected epidermal cells, day 4. n Cell death in senescing area, day 4. o Cell death in senescing area, day 7. a–c and f–o Light microscopy; d and e Fluorescence microscopy. d and e DCF-DA staining. f–j DAB staining. k–o Evans Blue staining. e Epidermis, m Mesophyll, s Stoma, t Trichome, v Vessel. Scale bars = 100 μm
Maximum production of H2O2 in senescing ‘non-bruised shreds’ was detected on day 4 and this did not change with the progression of deterioration until day 7 (Figs. 6i, j and 7). At the end of shelf life, however, H2O2 remained lower in senescing tissue than at the cut edge and at otherwise wounded tissue (Fig. 7). This suggested that with advancement of senescence at certain time point, the capacity of senescing cells to produce H2O2 might diminish.
Production of H2O2 in senescing and wounded lettuce fresh-cuts stored at 4 °C The amount of H2O2 is quantified by pixel intensity of DAB deposits. Initial value (day 0) is shown as dotted line. Presented values are means ± SEM (n–1), (n = 25). Quantification was done in 5 non-overlapping microscopy fields in each of at least 5 representative micrographs collected from 3 independent experiments; each separate experiment was carried out with fresh-cut samples prepared from 5 lettuce heads. Data indicated with same letters do not differ significantly from each other at P ≤ 0.05
The labelling with DCF-DA resulted in clearly distinguishable green fluorescence indicating substantial increase in the generation of overall ROS at the cut edge and in bruised sites (Fig. 5e, j). In comparison to fresh tissue at day 1 (Fig. 6d), ROS accumulation was spread over larger area by day 4 (Fig. 6e). These observations showed that enhanced generation of H2O2 and other ROS occurs close to the sites of injury and in senescing tissue. In general, the massive ROS, including H2O2, were detected in the cells most probably determined to die (Figs. 5b–d, g–i and 6i, j, n, o).
Electrolyte leakage
The measurement of electrolyte leakage showed that the cell death in wounded and senescing sites was accompanied with an increase in electrolyte leakage (Fig. 8). It proceeded concomitantly with chlorophyll loss, browning and the augmentation of H2O2 and overall ROS production. On day 1 of storage, the electrolyte leakage in all samples was still similar to that of the initial samples (day 0). At day 4 and day 7, electrolyte leakage was lowest in the senescing areas (showing yellowing) and highest in the wounded areas (cut edge and bruised sites, showing browning) (Fig. 8). These data indicated that senescing and wounded cells of lettuce fresh-cuts were undergoing cell death, of which the electrolyte leakage is well recognised marker of compromised membrane integrity.
Electrolyte leakage of tissue discs from wounded (cut edge and bruised sites) and non-wounded areas in lettuce fresh-cuts, stored at 4 °C Presented values are means ± SEM (n–1), (n = 9). At each time point, samples (15 leaf discs) were randomly taken from fresh-cuts from 3 boxes in three independent experiments with fresh-cuts prepared from 5 lettuce heads. Data indicated with same letters do not differ significantly from each other at P ≤ 0.05
Morphological occurrence of cell death
The dead cells were identified following the staining with Evans Blue and PI. Evans Blue positive dead cells were coloured in blue; PI positive nuclei in the dead cells emitted bright red fluorescence. After 1 day of storage, no dead cells were detected at the cut edge, bruised sites and non-wounded tissue (Figs. 5a, f and 6a, k). After 2 days of storage, cell death occurred in relatively low number of cells within the slightly browning area adjacent the wounded sites and this was accompanied with slightly elevated H2O2 level (Figs. 1a1, a2, a3).). Four days after cutting, most of the cells in the narrow zone close to the cut edge and at the sites of bruising were positive against Evans Blue and PI (Fig. 5b, c, g, h). In non-wounded areas of ‘non-bruised’ shreds, the cell death occurred throughout the entire senescing tissue (Fig. 6n, o) starting approximately a day later than in ‘bruised shreds’.
Propidium iodide-positive cells showed clear signs of condensed nuclei (Figs. 5c, h and 9d). Evans Blue staining revealed shrunken protoplasts separated from the cell walls both in the dead epidermal and in some of the dead mesophyll cells (Figs. 5g and 9b–c). Hydrogen peroxide remained within the shrunken protoplast (Fig. 9a). These features suggested necrotic type of PCD. Some of the cells appeared empty, Evans Blue negative (Fig. 9a–c). In the empty cells, H2O2 was not distinguishable by brown DAB deposits (Fig. 9a). The observations suggested that some of the cells might undergo vacuolar PCD, some might express necrotic and maybe others express mixed cell death phenotype.
Expression of programmed cell death phenotype in wounded and senescing cells in lettuce fresh-cuts, stored at 4 °C a Dead epidermal cells (with accumulated H2O2) at the cut surface, DAB staining; note the brown coloured protoplasts. b Dead epidermal cells in senescing fresh-cut, Evans Blue staining. c Dead mesophyll cells in senescing fresh-cut (epidermis removed), Evans Blue staining. d PI stained condensed nuclei (bright red fluorescence) in dead cells in senescing sites. a–c Note the shrunken protoplast retracted from the cell wall and b, d Condensed nuclei. a–c Some of the cells appear empty, DAB and Evans Blue negative. Samples were taken on day 4 of storage. cw Cell wall, dc Death cell, ec Empty cell, lc Living cell, n Nucleus, p Protoplast, t Trichome. Scale bars = 100 μm
Role of trichomes in wound-induced senescence
On day 1 of storage epidermal and mesophyll tissue of non-bruised fresh-cuts consisted of living cells (Fig. 6a, k) and the trichomes contained only living cells (Fig. 6b). On day 2, it was noticed that before any visible signs of senescence, the cells in the upper part of single trichomes in ‘non-bruised’ shreds were apparently dead (Evans Blue positive) (Fig. 6l). At the base of same trichomes, the cells were Evans Blue negative. After 4 days of storage at senescing sites, the number of entirely dead trichomes increased (Fig. 6c, m) and dead (blue coloured) cells appeared also in the connecting epidermal tissue (Fig. 6m). The cell death progressed (Fig. 6n) and on day 7 most of the cells in epidermis and parenchyma were dead (Fig. 6o). Cell death advancement in trichomes and connecting tissue (Fig. 6l, m) was accompanied with H2O2 accumulation (brown DAB deposits) that further spread over the whole senescing area (Fig. 6g–j). These observations suggested that in fresh-cuts, the trichomes at sites distant from the primary wound sites may be the first to respond to and propagate the long distance wound signal.
Discussion
Our earlier study suggested that wound stress-induced browning in lettuce fresh-cuts is associated with PCD symptoms (Iakimova and Woltering 2015). Here, we report further findings on morphological characterisation and signalling in wound-induced PCD. Most of the dead cells in the wounded and in non-injured (senescing) areas showed compacted nuclei and shrunken protoplast (retracted from cell wall). Some of the cells appeared empty suggesting that probably these are empty corpses remaining after autolysis of cellular content. Cell disappearance in the tissue undergoing highly advanced senescence substantiates the observations of Wagstaff et al. (2007) for vanishing cells in senescing detached leaves of postharvest lettuce heads. The observed cell death phenotypes resembling necrotic and vacuolar cell death classes suggested shared components of wound-induced cell death, senescence and HR PCD in lettuce. For example, phenotypic expression (e.g. protoplast shrinkage) of the dead cells together with activation of caspase 3-like protease and other elements of necrotic PCD are documented in pathogen challenged detached lettuce leaves and non-headed Chinese cabbage (Kiba et al. 2006, 2009; Li et al. 2006) whereas symptoms of vacuolar cell death are described in senescing lettuce leaves and in fresh-cuts subjected to high pressure stress (Wagstaff et al. 2007; Zhang et al. 2015). The changes in plasma membrane integrity and chlorophyll breakdown are markers of senescence and cell death in response to various stresses (Dhindsa et al. 1981; van Doorn and Woltering 2004; Lim et al. 2007; Song et al. 2012). Postharvest senescence in vegetable fresh-cuts suffering of storage-induced disorders, e.g. chilling injury, is also accompanied by membrane disruption and chlorophyll degradation (Artés et al. 2007; Hodges and Toivonen 2008; Saltveit 2002; Pedreschi and Lurie 2015; Pareek 2016). The observed in our experimental system increase in membrane permeability (electrolyte leakage) and chlorophyll loss indicate similarity between senescence and wound-induced cell death. The occurrence of dead cells of both phenotypes and the other detected physiological and biochemical markers of PCD in wounded and senescing tissue led to the assumption that part of the cells in lettuce fresh-cuts may undergo necrotic cell death and others may die in a manner of vacuolar cell death.
The biological role of the local wound-induced PCD in the vicinity of injured sites has been compared to the HR; both processes aimed at preventing the runaway spread of cell death (Cui et al. 2013; McCabe 2013). Our observations that cell death in lettuce fresh-cuts occurred quickly in the tissue close to the wound suggest that, in this system, PCD may function as a defence mechanism in order to rapidly seal-off the injury by physically separating the damaged from the healthy tissue with a layer of dead cells. In addition to the involvement of phenolics in the development of browning, it is thought that accumulation of polymerised phenolic compounds such as callose, suberin or lignin can play a role in building a physical barrier against propagating cell death response (Cui et al. 2013). Among the factors that are able to prevent the spreading of wound-induced death from the primary site of wounding throughout the rest of tissue is H2O2 which, apart from its role in PCD signalling (Levine et al. 1994; Jabs 1999; Neill et al. 2002), is involved also in pathways responsible for synthesis of antioxidant compounds that help the cells to cope with wound stress and can contribute to the strengthening of cell walls by participating in the cross-linking of its constituents (Cui et al. 2013; Tisi et al. 2008 and references therein).
Oxidative stress is involved in wound and other stress responses and is a substantial component of the PCD process (Jabs 1999; Sakamoto et al. 2005; Gill and Tuteja 2012). Hydrogen peroxide is recognised as localised mobile cell death factor in the HR PCD and in local wound signalling (Levine et al. 1994, 1996; León et al. 2001). We observed that the accumulation of H2O2 and other ROS corresponded to the advancement of browning and cell death at wounded sites. Obviously the oxygen species were produced in the wounded and in their neighbouring cells; additionally H2O2 may have diffused from the injured xylem vessels inside the area of cutting or bruising. This shows that ROS definitely contribute to the browning-associated confined wound response through mediating the cell death at the primary site of injury. Regarding the local cell death signalling, it is interesting to note that the presumed wound-induced primary signal molecules such as lysophospholipids (García et al. 2017) have previously been associated with the induction of cell death (necrotic PCD) in tomato cell cultures (Yakimova et al. 2006; Iakimova et al. 2013). The observed localised cell death at the wound site in fresh-cut lettuce may be triggered by these compounds. It addition, it is notable that a role of phosphatidylserine and its derivative lysophosphatidylserine is documented in animal cells undergoing apoptosis. These substances are involved in early apoptotic pathways and are also exposed on the outer surface of plasma membrane as a signal to phagocytes for recognising the apoptotic cells, thus promoting the phagocytosis—a process occurring in animal systems for removal of the remnants of apoptotic cells by macrophages (Denecker et al. 1999; Frasch and Bratton 2012).
The local browning, ROS production and cell death in wounded tissue occurred a day earlier than the first visible symptoms of senescence in non-wounded areas. In the later time points, ROS generation accompanied the course of senescence at the distant sites. Moreover, in comparison to the rapid increase of electrolyte leakage and chlorophyll loss at wounded sites, in non-injured tissue, these changes were delayed and expressed with lower severity. This suggested that ROS synthesis and the sequential senescence/cell death in non-wounded sites might be induced by a long-distance wound signal generated at the site of damage and transmitted toward the remote cells.
Potential players in long-distance stress communication, including wound signalling are jasmonic acid (JA), salicylic acid (SA), ethylene, NO, peptide messengers such as systemin, Ca2+-dependent pathways, MAPkinases, phospholipase A2, linoleic acid, octadecanoid pathways and electrical waves (López-Gálvez et al. 1996b; Ryan 2000; León et al. 2001; Campos-Vargas and Saltveit 2002; McCabe 2013). However, these factors might not operate collectively in the various stress situations and may function also as short-distance stress messengers. For example, Cui et al. (2013) assumed that wound-induced cell death is independent of SA, JA and ethylene but is related to abscisic acid (ABA). They found that ROS production is associated with ABA-stimulated local wound-induced cell death and showed that ABA additionally contributes to the spread of cell death away from the wound. The same authors also reported that the extent of dissemination of cell death is under control of the transcription factor BOTRYTIS SENSITIVE1/MYB108 which acts as a negative regulator of ABA production, hence preventing ABA-related long-distance wound signalling and limiting cell death to cells adjacent the wounds.
An intriguing question is how the senescence-inducing wound signalling was broadcasted to the unwounded parts of the lettuce fresh-cuts. We found that the first dead cells in non-injured areas appeared in trichomes. This was preceded by H2O2 accumulation in trichome cells. Further, H2O2 was intensively produced in the epidermal cells immediately connected to the respective trichomes and thereafter spread toward parenchyma. The cell death progression followed the same sequence. This suggested that trichomes might be the first structures distant from the wounded site that possibly can perceive a mobile signal generated in response to wound stress. In a model pathosystems, Wang et al. (2009) showed that trichomes can convey and incorporate external stress signals into intrinsic cellular pathways. These authors demonstrated that treatment of trichomes in tobacco leaves with the protein elicitor ParA1 from Phytophthora parasitica var. nicotianae activated HR PCD pathways occurring sequentially in trichomes and in the epidermal and mesophyll cells. An increase of H2O2 level was detected initially in the upper cell of trichome; next the H2O2 appeared in the lower trichome cells and thereafter in the connected epidermal and neighbouring mesophyll tissue. Our observations showed similar order of H2O2 production first in trichomes and later in the epidermal and mesophyll cells. This provides information suggesting that trichomes might participate in wound response of lettuce fresh-cuts by sensing and transmitting a long-distance wound signal which stimulates oxidative stress and additional events leading to cellular senescence (PCD). In support to our assumption is the discovery that Arabidopsis thaliana trichomes may function as mechanosensory system by sensing even slight folding, bruising, pressing or vibrations caused by insects touching the leaf surface or flying over it. Such disturbances were shown to induce changes in cytosolic Ca2+ and shift of pH toward alkaline state which in turn contributes to activation of pathways related to synthesis of plant defence toxins (Zhou et al. 2017). Another work suggested that tomato glandular trichomes can detect physical activity on the leaf surface and activate JA and H2O2 associated processes (Tooker et al. 2010). In velvet bean (Mucuna pruriens), insect-provoked mild mechanical stress has stimulated gene expression of a protein, containing domains belonging to papain family of cysteine proteases (Singh and Dhawan 2017). These proteases are known to be involved in diverse defence responses, HR and other PCD processes (Woltering 2010). The putative mobile signal that may potentiate senescence/cell death cascade at sites remote from the wounded lettuce tissue remains to be further elucidated.
Conclusions
Our previous and current studies soundly indicate that PCD is an integral part of wound-associated browning disorder in lettuce fresh-cuts. Here, we present detailed characteristic of morphological, physiological and biochemical processes underlying the wound PCD response in this vegetable model. The morphological features of the dead cells (shrunken protoplasts and condensed nuclei, but also the appearance of empty corpses), together with H2O2 and overall ROS accumulation, compromised integrity of cellular membrane (electrolyte leakage) and presumable photosynthesis decline (chlorophyll loss) in both wounded and senescing sites pointed to cell death resembling a mixture of necrotic and vacuolar PCD types. The quick occurrence of cell death in vicinity of the wounds suggested that PCD may contribute to restricting the damage to the primary site of wounding by serving as a mechanism for building a physical barrier of dead cells between the injured and healthy tissue. The wound stress accelerated senescence in non-wounded tissue most probably through long-distance wound signalling. Trichomes in non-wounded sites were the first to show H2O2 accumulation and cell death followed by cell death in connecting epidermal tissue and consecutive senescence over larger area. This suggested a possible role of trichomes in mediating senescence/cell death at sites remote from the wounds (Fig. 10).
Schematic illustration of PCD involvement in the wound response in fresh-cut lettuce. Wounding (at the cut edge or bruised sites) involves the production of lysophospholipids (such as LPA, lysophosphatidic acid; LPS; lysophosphatidylserine; LPI, lysophosphatidylinositol) and causes rapid browning confined to the area surrounding the injured tissue. Browning is associated with massive cell death, H2O2 and general ROS accumulation, electrolyte leakage and chlorophyll loss. Dead cells mostly resemble necrotic PCD phenotype (shrunken protoplast). Dying cells generate signal molecules that travel over greater distances to cause ROS and cell death at distant sites; first in trichomes and subsequently in the connecting epidermal and mesophyll cells. In addition to necrotic PCD also vacuolar PCD (leaving empty cell corpses behind) and the complete disappearance of cells are observed. cw Cell wall, n Nucleus, pp. Protoplast
The findings add new information for the role of PCD in wound response in lettuce fresh-cuts and may open a path toward studies for controlling the deterioration in postharvest leafy vegetables through specifically targeting the PCD events.
References
Artés F, Gómez PA, Artés-Hernández F (2007) Physical, physiological and microbial deterioration of minimally fresh processed fruits and vegetables. Food Sci Technol Int 13(3):177–188. https://doi.org/10.1177/1082013207079610
Ballantyne A, Stark R, Selman JD (1988) Modified atmosphere packaging of shredded lettuce. Int J Food Sci Technol 23:267–274. https://doi.org/10.1111/j.1365-2621.1988.tb00578.x
Bolin HR, Stafford AE, King JRAD, Huxsoll CC (1997) Factors affecting the storage stability of shredded lettuce. J Food Sci 42:1319–1321. https://doi.org/10.1111/j.1365-2621.1977.tb14487.x
Buchanan-Wollaston V, Earl S, Harrison E, Mathas E, Navabpour S, Page T, Pink D (2003) The molecular analysis of leaf senescence—a genomics approach. Plant Biotechnol J 1:3–22. https://doi.org/10.1046/j.1467-7652.2003.00004.x
Campos-Vargas R, Saltveit MF (2002) Involvement of putative chemical wound signals in the induction of phenolic metabolism in wounded lettuce. Physiol Plantarum 114:73–84. https://doi.org/10.1034/j.1399-3054.2002.1140111.x
Cantre D, Herremans E, Verboven P, Ampofo-Asiama J, Hertog MLATM, Nicolaï BM (2017) Tissue breakdown of mango (Mangifera indica L. cv. Carabao) due to chilling injury. Postharvest Biol Technol 125:99–111. https://doi.org/10.1016/j.postharvbio.2016.11.009
Cantwell MI, Suslow TV (2002) Fresh-cut fruits and vegetables. Aspects of physiology, preparation and handling that affect quality. In: Kader AA (ed) Postharvest Technology of Horticultural Crops. Division of Agriculture and Natural Resources. Publication 3311. Davis, US: University of California, pp. 445–463
Castañer M, Gil MI, Artés F, Tomas-Barberan FA (1996) Inhibition of browning of harvested head lettuce. J Food Sci 61(2):314–316. https://doi.org/10.1111/j.1365-2621.1996.tb14184.x
Coupe SA, Sinclair BK, Watson M, Heyes JA, Eason JR (2003) Identification of dehydration-responsive cysteine proteases during postharvest senescence of broccoli florets. J Exp Bot 54:1045–1056. https://doi.org/10.1093/jxb/erg105
Coupe SA, Watson LM, Ryan DJ, Pinkney TT, Eason JR (2004) Molecular analysis of programmed cell death during senescence in Arabidopsis thaliana and Brassica oleracea, cloning broccoli LSD1, Bax inhibitor and serine palmityltransferase homologues. J Exp Bot 55:59–68. https://doi.org/10.1093/jxb/erh018
Couture R, Cantwel MI, Ke D, Saltveit ME (1993) Physiological attributes related to quality attributes and storage life of minimally processed lettuce. HortSci 58:609–610
Cui F, Brosché M, Sipari N, Tang S, Overmyer K (2013) Regulation of ABA dependent wound-induced spreading cell death by MYB108. New Phytol 200:634–640. https://doi.org/10.1111/nph.12456
Degl'Innocenti E, Pardossi A, Tognoni F, Guidi L (2007) Physiological basis of sensitivity to enzymatic browning in ‘lettuce’, ‘escarole’ and ‘rocket salad’ when stored as fresh-cut products. Food Chem 104:209–215. https://doi.org/10.1016/j.foodchem.2006.11.026
Denecker G, Dooms H, Van Loo G, Vercammen D, Grooten J, Fiers W et al (1999) Phosphatidylserine exposure during apoptosis precedes release of cytochrome c and decrease in mitochondrial transmembrane potential. FEBS Lett 465:47–52. https://doi.org/10.1016/S0014-5793(99)01702-0
Dhindsa RS, Plumb-Dhindsa P, Thorpe TA (1981) Leaf senescence: correlated with increased levels of membrane permeability and lipid peroxidation, and decreased levels of superoxide dismutase and catalase. J Exp Bot 32(126):93–101. https://doi.org/10.1093/jxb/32.1.93
Eason JR, West PJ, Brummell DA, Watson LM, Somerfield SD, McLachlan ARG (2014) Overexpression of the protease inhibitor BoCPI-1 in broccoli delays chlorophyll loss after harvest and causes down-regulation of cysteine protease gene expression. Postharvest Biol Technol 97:23–31. https://doi.org/10.1016/j.postharvbio.2014.06.006
Fernández-Trujillo JP, Martínez JA (2006) Ultrastructure of the onset of chilling injury in cucumber fruit. J Appl Bot Food Quality 80:100–110
Fonseca SC, Oiveira FAR, Brecht JK (2002) Modelling respiration rate of fresh fruits and vegetables for modified atmosphere packages: a review. J Food Eng 52:99–119. https://doi.org/10.1016/S0260-8774(01)00106-6
Frasch SC, Bratton DI (2012) Emerging roles for lysophosphatidylserine in resolution of inflammation. Prog Lipid Res 51(3):199–207. https://doi.org/10.1016/j.plipres.2012.03.001
Gapper NE, Coupe SA, McKenzi MJ, Sinclair BK, Lill RE, Jameson PE (2005) Regulation of harvest-induced senescence in broccoli (Brassica oleracea var. Italica) by cytokinin, ethylene, and sucrose. J Plant Growth Regul 24:153–165. https://doi.org/10.1007/s00344-005-0028-8
García CJ, García-Villalba R, Gil MI, Tomas-Barberan FA (2017) LC-MS untargeted metabolomics to explain the signal metabolites inducing browning in fresh-cut lettuce. J Agric Food Chem 65:4526–4535. https://doi.org/10.1021/acs.jafc.7b01667
Gil MI, Tudela JA, Martínez-Sánchez A, Luna MC (2012) Harvest maturity indicators of leafy vegetables. Stewart Postharvest Review 8:1–9(9). https://doi.org/10.2212/spr.2012.1.1
Gill SS, Tuteja N (2012) Reactive oxygen species and antioxidant machinery in abiotic stress tolerance in crop plants. Plant Physiol Biochem 48:909–930. https://doi.org/10.1016/j.plaphy.2010.08.016
Gunawardena AHLAN, Pearce DM, Jackson MB, Hawes CR, Evans DE (2001) Characterization of programmed cell death during aerenchyma formation induced by ethylene or hypoxia in roots of maize (Zea mays L.) Planta 212:205–214. https://doi.org/10.1007/s004250000381
Henzi MX, Christey MC, McNeil DL (2000) Morphological characterisation and agronomic evaluation of transgenic broccoli (Brassica oleracea L. var. italica) containing an antisense ACC oxidase gene. Euphytica 113:9–18. https://doi.org/10.1023/A:1003979801348
Hodges M, Toivonen PMA (2008) Quality of fresh-cut fruits and vegetables as affected by exposure to abiotic stress. Postharvest Biol Technol 48(2):155–162. https://doi.org/10.1016/j.postharvbio.2007.10.016
Hurr BM, Huber DJ, Vallejos CE (2010) Features of programmed cell death precede watersoaking development in ethylene-treated immature cucumber fruit. Postharvest Biol Technol 58:13–20. https://doi.org/10.1016/j.postharvbio.2010.03.011
Iakimova ET, Woltering EJ (2015) Nitric oxide prevents wound-induced browning and delays senescence through inhibition of hydrogen peroxide accumulation in fresh-cut lettuce. IFSET 30:157–169. https://doi.org/10.1016/j.ifset.2015.06.001
Iakimova ET, Woltering EJ (2017) Xylogenesis in zinnia (Zinnia elegans) cell cultures: unravelling the regulatory steps in a complex developmental programmed cell death event. Planta 245:681–705. https://doi.org/10.1007/s00425-017-2656-1
Iakimova ET, Michaeli R, Woltering EJ (2013) Involvement of phospholipase D-related signal transduction in chemical-induced programmed cell death in tomato cell cultures. Protoplasma 250(5):1169–1183. https://doi.org/10.1007/s00709-013-0497-8
Jabs T (1999) Reactive oxygen intermediates as mediators of programmed cell death in plants and animals. Biochem Pharmacol 57:231–245. https://doi.org/10.1016/S0006-2952(98)00227-5
Kader AA, Lipton WJ, Morris LL (1973) Systems for scoring quality of harvested lettuce. HortSci 8(5):408–409
Keogh RC, Deverall BJ, Mcleod S (1980) Comparison of histological and physiological responses to Phakopsora pachyrhizi in resistant and susceptible soybean. Trans Br Mycol Soc 74:329–333. https://doi.org/10.1016/S0007-1536(80)80163-X
Kiba A, Sangawa Y, Ohnishi K, Yao N, Park P, Nakayashiki H, Tosa Y, Mayama S, Hikichi Y (2006) Induction of apoptotic cell death leads to the development of bacterial rot caused by Pseudomonas cichorii. MPMI 19:112–122. https://doi.org/10.1094/MPMI-19-0112
Kiba A, Lee KY, Ohnishi K, Park P, Nakayashiki H, Tosa Y, Mayama S, Hikichi Y (2009) Induction of reactive oxygen generation and functional changes in mitochondria and their involvement in the development of bacterial rot in lettuce caused by Pseudomonas cichorii. Physiol Mol Plant Pathol 74:45–54. https://doi.org/10.1016/j.pmpp.2009.08.006
Lam E (2004) Controlled cell death, plant survival and development. Nat Rev Mol Cell Biol 5:305–315. https://doi.org/10.1038/nrm1358
León J, Rojo E, Sánchez-Serrano JJ (2001) Wound signaling in plants. J Exp Bot 52(354):1–9. https://doi.org/10.1093/jexbot/52.354.1
Levine A, TenhakenR DR, Lamb CJ (1994) Н2О2 from the oxidative burst orchestrates the plant hypersensitive response. Cell 79:583–593. https://doi.org/10.1016/0092-8674(94)90544-4
Levine A, Pennell RI, Alvarez ME, Palmer R, Lamb CJ (1996) Calcium-mediated apoptosis in plant hypersensitive disease resistance response. Curr Biol 6:427–437. https://doi.org/10.1016/S0960-9822(02)00510-9
Li J, Zhang Z-G, Ji R, Wang Y-C, Zheng X-B (2006) Hydrogen peroxide regulates elicitor PB90-induced cell death and defense in non-heading Chinese cabbage. Physiol Mol Plant Path 67:220–230. https://doi.org/10.1016/j.pmpp.2006.02.002
Li S-P, Hu K-D, Hu L-Y, Li Y-H, Jiang A-M, Xiao F, Han Y, Liu YS, Zhang H (2014) Hydrogen sulfide alleviates postharvest senescence of broccoli by modulating antioxidant defense and senescence-related gene expression. J Agr Food Chem 62:1119–1129. https://doi.org/10.1021/jf4047122
Lim PO, Kim HJ, Nam HG (2007) Leaf senescence. Annu Rev Plant Biol 58:115–136. https://doi.org/10.1146/annurev.arplant.57.032905.105316
López-Gálvez G, Saltveit M, Cantwell M (1996a) The visual quality of minimally processed lettuces stored in air or controlled atmosphere with emphasis on romaine and iceberg types. Postharvest Biol Technol 8(3):179–190. https://doi.org/10.1016/0925-5214(95)00002-X
López-Gálvez G, Saltveit M, Cantwell M (1996b) Wound-induced phenylalanine ammonia lyase activity: factors affecting its induction and correlation with the quality of minimally processed lettuces. Postharvest Biol Technol 9:223–233. https://doi.org/10.1016/S0925-5214(96)00050-6
Mahajan PV, Caleb OJ, Singh Z, Watkins CB, Geyer M (2014) Postharvest treatments of fresh produce. Philos T R Soc A 372:20130309. https://doi.org/10.1098/rsta.2013.0309
Martínez-Sánchez A, Luna MC, Selma MV, Tudela JA, Abad J, Gil MI (2012) Baby-leaf and multi-leaf of green and red lettuces are suitable raw materials for the fresh-cut industry. Postharvest Biol Technol 63:1–10. https://doi.org/10.1016/j.postharvbio.2011.07.010
McCabe PF (2013) Healing and closure following death: death signals from a wounded leaf. New Phytol 200(3):590–591. https://doi.org/10.1111/nph.12527
McCabe MS, Garratt LC, Schepers F, Jordi WJRM, Stoopen GM, Davelaar E et al (2001) Effects of PSAG12-IPT gene expression on development and senescence in transgenic lettuce. Plant Physiol 127:505–516. https://doi.org/10.1104/pp.010244
Mur LAJ, Kenton P, Lloyd AJ, Ougham H, Prats E (2008) The hypersensitive response; the centenary is upon us but how much do we know? J Exp Bot 59:501–520. https://doi.org/10.1093/jxb/erm239
Neill SJ, Desikan R, Clarke A, Hurst RD, Hancock JT (2002) Hydrogen peroxide and nitric oxide as signalling molecules in plants. J Exp Bot 53:1237–1247. https://doi.org/10.1093/jexbot/53.372.1237
Pareek S (2016) Fresh-cut fruits and vegetables: technology, physiology, and safety. Pareek (ed) part 1, chapter 1, 1st edn. CRC press, Taylor and Francis Group, Boca Ration, Florida, 623 pp
Pedreschi R, Lurie S (2015) Advances and current challenges in understanding postharvest abiotic stresses in perishables. Postharvest Biol Technol 107:77–89. https://doi.org/10.1016/j.postharvbio.2015.05.004
Pennell RI, Lamb C (1997) Programmed cell death in plants. Plant Cell 9(7):1157–1168 http://www.jstor.org/stable/41433787
Pereyra L, Roura SI, del Valle CE (2005) Phenylalanine ammonia lyase activity in minimally processed Romaine lettuce. Food Sci Technol 38:67–72. https://doi.org/10.1016/j.lwt.2004.05.004
Price AM, Orellana DFA, Salleh FM, Stevens R, Acock R, Buchanan-Wollaston V et al (2008) A comparison of leaf and petal senescence in wallflower reveals common and distinct patterns of gene expression and physiology. Plant Physiol 147:1898–1912. https://doi.org/10.1104/pp.108.120402
Quirino BF, Noh Y-S, Himelblau E, Amasino RM (2000) Molecular aspects of leaf senescence. Trends Plant Sci 5(7):278–282. https://doi.org/10.1016/S1360-1385(00)01655-1
Reape TJ, Molony EM, McCabe PF (2008) Programmed cell death in plants: distinguishing between different modes. J Exp Bot 59:435–444. https://doi.org/10.1093/jxb/erm258
Rico D, Martín-Diana AB, Henehan J, Frías GTM, Barry-Ryan C (2006) Effect of ozone and calcium lactate treatments on browning and textured properties of freshcut lettuce. J Sci Food Agric 86:2179–2188. https://doi.org/10.1002/jsfa.2594
Ryan CA (2000) The systemin signaling pathway: differential activation of plant defensive genes. BBA, Protein Struct Mol Enzymol 1477:112–121. https://doi.org/10.1016/S0167-4838(99)00269-1
Sakamoto M, Tada Y, Nakayashiki H, Tosa Y, Mayama S (2005) Two phases of intracellular reactive oxygen species production during victorin-induced cell death in oats. J GenPlant Path 71:387–394. https://doi.org/10.1007/s10327-005-0220-5
Saltveit ME (2002) The rate of ion leakage from chilling-sensitive tissue does not immediately increase upon exposure to chilling temperatures. Postharvest Biol Technol 26:295–304. https://doi.org/10.1016/S0925-5214(02)00049-2
Saltveit ME (2018) Anaerobic exposure before or after wounding reduces the production of wound-induced phenolic compounds in fresh-cut lettuce. Postharvest Biol Technol 135:77–82. https://doi.org/10.1016/j.postharvbio.2017.08.022
Saltveit ME, Choi Y-J (2007) Aromatic- and di-carboxylates inhibit wound-induced phenolic accumulation in excised lettuce (Lactuca sativa L.) leaf tissue. Postharvest Biol Technol 46:222–229. https://doi.org/10.1016/j.postharvbio.2007.05.004
Shibuya K, Yamada T, Ishimura K (2016) Morphological changes in senescing petal cells and the regulatory mechanism of petal senescence. J Exp Bot 67:5909–5918. https://doi.org/10.1093/jxb/erw337
Singh SK, Dhawan SS (2017) Analyzing trichomes and spatio-temporal expression of a cysteine protease gene Mucunain in Mucuna pruriens L. (DC). Protoplasma. https://doi.org/10.1007/s00709-017-1164-2
Song JY, Kim DS, Lee M-C, Lee KJ, Kim J-B, Kim SH et al (2012) Physiological characterization of gamma-ray induced salt tolerant rice mutants. Australian. J Crop Sci 6(3):421–429
Thordal-Christensen H, Zhang Z, Wei Y, Collinge DB (1997) Subcellular localization of H2O2 in plants. H2O2 accumulation in papillae and hypersensitive response during the barley-powdery mildew interaction. Plant J 11:1187–1194. https://doi.org/10.1046/j.1365-313X.1997.11061187.x
Tisi A, Angelini R, Cona A (2008) Wound healing in plants. Plant Signal Behav 3(3):204–206
Tooker J, Peiffer M, Luthe DS, Felton GW (2010) Trichomes as sensors. Plant Signal Behav 5(1):73–75. https://doi.org/10.4161/psb.5.1.10234
van Doorn WG, Woltering EJ (2004) Senescence and programmed cell death: substance or semantics? J Exp Bot 55:2147–2153. https://doi.org/10.1093/jxb/erh264
van Doorn WG, Woltering EJ (2005) Many ways to exit? Cell death categories in plants. Trends in Plant Sci 10:117–122. https://doi.org/10.1016/j.tplants.2005.01.006
van Doorn WG, Woltering EJ (2008) Physiology and molecular biology of petal senescence. J Exp Bot 59:453–480. https://doi.org/10.1093/jxb/erm356
van Doorn WG, Woltering EJ (2010) What about the role of autophagy in PCD? Trends Plant Sci 15:361–362. https://doi.org/10.1016/j.tplants.2010.04.009
van Doorn WG, Beers EP, Dangl JL, Franklin-Tong VE, Gallois P, Hara-Nishimura I, Jones AM, Kawai-Yamada M, Lam E, Mundy J, Mur LAJ, Petersen M, Smertenko A, Taliansky M, van Breusegem F, Wolpert T, Woltering E, Zhivotovsky B, Bozhkov PV (2011) Morphological classification of plant cell deaths. Cell Death Differ 18:1241–1246. https://doi.org/10.1038/cdd.2011.36
Wagstaff C, Clarkson GJJ, Rothwell SD, Page A, Taylor G, Dixon MS (2007) Characterisation of cell death in bagged baby salad leaves. Postharvest Biol Technol 46:150–159. https://doi.org/10.1016/j.postharvbio.2007.04.013
Wang Y, Liu R, Chen L, Wang Y, Liang Y, Wu X, Li B, Wu J, Liang Y, Wang X, Zhang C, Wang Q, Hong X, Dong H (2009) Nicotiana tabacum TTG1 contributes to ParA1- induced signalling and cell death in leaf trichomes. J Cell Sci 122:2673–2685. https://doi.org/10.1242/jcs.049023
Witkowska IM, Woltering EJ (2013) Plant age affects wound-induced senescence in Lactuca sativa L. J plant. Biochem Physiol 2:119. https://doi.org/10.4172/2329-9029.1000119
Witkowska IM, Woltering EJ (2014) Storage of intact heads prior to processing limits the shelf-life of fresh-cut Lactuca sativa L. Postharvest Biol Technol 91:25–31. https://doi.org/10.1016/j.postharvbio.2013.12.011
Woltering EJ (2010) Death proteases: alive and kicking. Trends Plant Sci 15(4):185–188. https://doi.org/10.1016/j.tplants.2010.02.001
Woltering EJ, Iakimova ET (2010) Programmed cell death and postharvest deterioration of horticultural produce. Acta Hortic (877):991–998. https://doi.org/10.17660/ActaHortic.2010.877.133
Woltering EJ, Seifu YW (2015) Low intensity monochromatic red, blue or green light increases the carbohydrate levels and substantially extends the shelf life of fresh-cut lettuce. Acta Hortic (1079):257–264. https://doi.org/10.17660/ActaHortic.2015.1079.30
Yakimova ET, Kapchina-Toteva VM, Laarhoven L-J, Harren FM, Woltering EJ (2006) Involvement of ethylene and lipid signalling in cadmium-induced programmed cell death in tomato suspension cells. Plant Physiol Biochem 44(10):581–589. https://doi.org/10.1016/j.plaphy.2006.09.003
Yen C-H, Yang C-H (1998) Evidence for programmed cell death during leaf senescence in plants. Plant Cell Physiol 39(9):922–927
Zhang L, Yao J, Zhang Y, Liao X, Chen F, Hu X (2015) Microstructural and morphological behaviors of asparagus lettuce cells subject to high pressure processing. Food Res Int 71:174–183. https://doi.org/10.1016/j.foodres.2015.01.036
Zhou LH, Liu SB, Wang PF, Lu TJ, Xu F, Genin GM, Pickard BG (2017) The Arabidopsis trichome is an active mechanosensory switch. Plant Cell Environ 40:611–621. https://doi.org/10.1111/pce.12728
Funding
This work was supported by the Netherlands Organization for Scientific Research (NWO), Visitor travel grant number 040.11.306 and partially by Agricultural Academy of Bulgaria, Project P175/2017 and, EC Erasmus+ Programme, KA1, Mobility for learners and staff (2016-2017).
Author information
Authors and Affiliations
Contributions
E.T. Iakimova performed the experimental research, data processing, presentation and discussion, and drafted the manuscript. E.J. Woltering designed and coordinated the study and contributed to presentation and discussion of the results and manuscript writing.
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Additional information
Handling Editor: Klaus Harter
Rights and permissions
Open Access This article is distributed under the terms of the Creative Commons Attribution 4.0 International License (http://creativecommons.org/licenses/by/4.0/), which permits unrestricted use, distribution, and reproduction in any medium, provided you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made.
About this article
Cite this article
Iakimova, E.T., Woltering, E.J. The wound response in fresh-cut lettuce involves programmed cell death events. Protoplasma 255, 1225–1238 (2018). https://doi.org/10.1007/s00709-018-1228-y
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00709-018-1228-y