Abstract
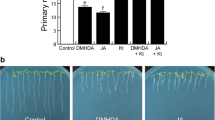
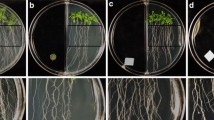
Plant growth-promoting rhizobacteria are natural inhabitants of roots, colonize diverse monocot and dicot species, and affect several functional traits such as root architecture, adaptation to adverse environments, and protect plants from pathogens. N,N-dimethyl-hexadecylamine (C16-DMA) is a rhizobacterial amino lipid that modulates the postembryonic development of several plants, likely as part of volatile blends. In this work, we evaluated the bioactivity of C16-DMA and other related N,N-dimethyl-amines with varied length and found that inhibition of primary root growth was related to the length of the acyl chain. C16-DMA inhibited primary root growth affecting cell division and elongation, while promoting lateral root formation and root hair growth and density in Arabidopsis thaliana (Arabidopsis) wild-type (WT) seedlings. Interestingly, C16-DMA induced the expression of the jasmonic acid (JA)-responsive gene marker pLOX2:uidA, while JA-related mutants jar1, coi1-1, and myc2 affected on JA biosynthesis and perception, respectively, are compromised in C16-DMA responses. Comparison of auxin-regulated gene expression, root architectural changes in WT, and auxin-related mutants aux1-7, tir1/afb2/afb3, and arf7-1/arf19-1 to C16-DMA shows that the C16-DMA effects occur independently of auxin signaling. Together, these results reveal a novel class of aminolipids modulating root organogenesis via crosstalk with the JA signaling pathway.










Similar content being viewed by others
References
Beraldi-Campesi H (2013) Early life on land and the first terrestrial ecosystems. Ecol Process 2:1–17. doi:10.1186/2192-1709-2-1
Blancaflor EB, Hou G, Chapman KD (2003) Elevated levels of N-lauroylethanolamine, an endogenous constituent of desiccated seeds, disrupt normal root development in Arabidopsis thaliana seedlings. Planta 217:206–217. doi:10.1007/s00425-003-0985-8
Camilli A, Bassler BL (2006) Bacterial small-molecule signaling pathways. Science 311:1113–1116. doi:10.1126/science.1121357
Campos-Cuevas JC, Pelagio-Flores R, Raya-González J, Méndez-Bravo A, Ortiz-Castro R, López-Bucio J (2008) Tissue culture of Arabidopsis thaliana explants reveals a stimulatory effect of alkamides on adventitious root formation and nitric oxide accumulation. Plant Sci 174:165–173. doi:10.1016/j.plantsci.2007.11.003
Castulo-Rubio DY, Alejandre-Ramírez NA, Orozco-Mosqueda MC, Santoyo G, Macías-Rodríguez LI, Valencia-Cantero E (2015) Volatile organic compounds produced by the rhizobacterium Arthrobacter agilis UMCV2 modulate Sorghum bicolor (strategy II plant) morphogenesis and SbFRO1 transcription in vitro. J Plant Growth Regul 34:611–623. doi:10.1007/s00344-015-9495-8
Chapman KD, Tripathy S, Venables B, Desouza AD (1998) N-acylethanolamines: formation and molecular composition of a new class of plant lipids. Plant Physiol 116:1163–1168
Dharmasiri N, Dharmasiri S, Weijers D, Lechner E, Yamada M, Hobbie L, Ehrismann J, Jürgens G, Estelle M (2005) Plant development is regulated by a family of auxin receptor F box proteins. Dev Cell 9:109–119. doi:10.1016/j.devcel.2005.05.014
Feys BJF, Benedetti CE, Penfold CN, Turner JG (1994) Arabidopsis mutants selected for resistance to the phytotoxin coronatine are male-sterile, insensitive to methyl jasmonate, and resistant to a bacterial pathogen. Plant Cell 6:751–759. doi:10.1105/tpc.6.5.751
Hartmann A, Schikora A (2012) Quorum sensing of bacteria and trans-kingdom interactions of N-acyl homoserine lactones with eucaryotes. J Chem Ecol 38:704–713. doi:10.1007/s10886-012-0141-7
Hernández-León R, Rojas-Solís D, Contreras-Pérez M, Orozco-Mosqueda MC, Macías-Rodríguez LI, Reyes-de la Cruz H, Valencia-Cantero E, Santoyo G (2015) Characterization of the antifungal and plant growth-promoting effects of diffusible and volatile organic compounds produced by Pseudomonas fluorescens strains. Bio Control 81:83–92. doi:10.1016/j.biocontrol.2014.11.011
Jensen AB, Raventos D, Mundy J (2002) Fusion genetic analysis of jasmonate signalling mutants in Arabidopsis. Plant J 29:595–606. doi:10.1046/j.0960-7412.2001.01241.x
Keller L, Surette MG (2006) Communication in bacteria: an ecological and evolutionary perspective. Nat Rev Microbiol 4:249–258. doi:10.1038/nrmicro1383
Liu W, Wei M, Bingyu Z, Feng L (2008) Antifungal activities and components of VOCs produced by Bacillus subtilis G8. Curr Res Bacteriol 1:28–34
López-Bucio J, Millán-Godínez M, Méndez-Bravo A, Morquecho-Contreras A, Ramírez-Chávez E, Molina-Torres J, Pérez-Torres A, Higuchi M, Kakimoto T, Herrera-Estrella L (2007) Cytokinin receptors are involved in alkamide regulation of root and shoot development in Arabidopsis thaliana. Plant Physiol 145:1703–1713. doi:10.1104/pp.107.107953
Mathesius U, Mulders S, Gao M, Teplitski M, Caetano-Anollés G, Rolfe BG, Bauer WD (2003) Extensive and specific responses of a eukaryote to bacterial quorum-sensing signals. Proc Natl Acad Sci U S A 100:1444–1449. doi:10.1073/pnas.262672599
Méndez-Bravo A, Raya-González J, Herrera-Estrella L, López-Bucio J (2010) Nitric oxide is involved in alkamide-induced lateral root development in Arabidopsis. Plant Cell Physiol 51:1612–1626. doi:10.1093/pcp/pcp117
Morquecho-Contreras A, López-Bucio J (2007) Cannabinoid-like signalling and other new developmental pathways in plants. Int J Plant Dev Biol 1:34–41
Morquecho-Contreras A, Méndez-Bravo A, Pelagio-Flores R, Raya-González J, Ortiz-Castro R, López-Bucio J (2010) Characterization of drr1, an alkamide resistant mutant of Arabidopsis reveals an important role for small lipid amides in lateral root development and plant senescence. Plant Physiol 152:1659–1673. doi:10.1104/pp.109.149989
Orozco-Mosqueda MC, Macías-Rodríguez LI, Santoyo G, FloresCortez I, Farías-Rodríguez R, Valencia-Cantero E (2013) Medicago truncatula increases its Fe-uptake mechanisms in response to volatile organic compounds produced by Sinorhizobium meliloti. Folia Microbiol 58:579–585. doi:10.1007/s12223-013-0243-9013-0243-9
Ortiz-Castro R, Martínez-Trujillo M, López-Bucio J (2008) N‐acyl‐L‐homoserine lactones: a class of bacterial quorum‐sensing signals alter post‐embryonic root development in Arabidopsis thaliana. Plant Cell Environ 31:1497–1509. doi:10.1111/j.1365-3040.2008.01863.x
Ortiz-Castro R, Méndez-Bravo A, López-Bucio J (2010) Amino compound-containing lipids: a novel class of signals regulating plant development. In: Heidelberg (ed) Plant developmental biology-biotechnological perspectives, 1st Ed. Springer, Berlin, pp 209–226. doi: 10.1007/978-3-642-04670-4_11
Ortiz-Castro R, Díaz-Pérez C, Martínez-Trujillo M, Rosa E, Campos-García J, López-Bucio J (2011) Transkingdom signaling based on bacterial cyclodipeptides with auxin activity in plants. Proc Natl Acad Sci U S A 108:7253–7258. doi:10.1073/pnas.1006740108
Parry G, Calderon-Villalobos LI, Prigge M, Peret B, Dharmasiri S, Itoh H, Lechner E, Gray WM, Bennett M, Estelle M (2009) Complex regulation of the TIR1/AFB family of auxin receptors. Proc Natl Acad Sci U S A 106:22540–22545. doi:10.1073/pnas.0911967106
Picket FB, Wilson AK, Estelle M (1990) The aux1 mutation of Arabidopsis confers both auxin and ethylene resistance. Plant Physiol 94:1462–1466. doi:10.1104/pp.94p3.1462
Ramírez-Chávez E, López-Bucio J, Herrera-Estrella L, Molina-Torres J (2004) Alkamides isolated from plants promote growth and alter root development in Arabidopsis. Plant Physiol 134:1058–1068. doi:10.110/pp.103.034553
Raya-González J, Pelagio-Flores R, López-Bucio J (2012) The jasmonate receptor COI1 plays a role in jasmonate-induced lateral root formation and lateral root positioning in Arabidopsis thaliana. J Plant Physiol 169:1348–1358. doi:10.1016/j.jplph.2012.05.002
Raya-González J, Hernández-Abreu E, Valencia-Cantero E, López-Bucio J (2016) Microbial resources for improved crop productivity. In: Gupta VK, Sharma GD, Tuohy MG, Gaur R (eds) The handbook of microbial bioresources. CAB International, Boston, pp 1–13
Ryu CM, Farag MA, Hu CH, Reddy MS, Wei HX, Paré PW, Kloepper JW (2003) Bacterial volatiles promote growth in Arabidopsis. Proc Natl Acad Sci U S A 100:4927–4932. doi:10.1073/pnas.0730845100
Santelia D, Vincenzetti V, Azzarello E, Bovet L, Fukao Y, Düchtig P, Mancuso S, Martinoia E, Geisler M (2005) MDR‐like ABC transporter AtPGP4 is involved in auxin‐mediated lateral root and root hair development. FEBS Lett 579:5399–5406. doi:10.1016/j.febslet.2005.08.061
Santner A, Calderón-Villalobos I, Estelle M (2009) Plant hormones are versatile chemical regulators of plant growth. Nat Chem Biol 5:301–307. doi:10.1038/nchembio.165
Schikora A, Schenk ST, Hartmann A (2016) Beneficial effects of bacteria-plant communication based on quorum-sensing molecules of the N-acyl homoserine lactone group. Plant Mol Biol 6:605–612. doi:10.1007/s11103-016-0457-8
Staswick PE, Su W, Howell SH (1992) Methyl jasmonate inhibition of root growth and induction of a leaf protein are decreased in an Arabidopsis thaliana mutant. Proc Natl Acad Sci U S A 89:6837–6840. doi:10.1073/pnas.89.15.6837
Sun J, Xu Y, Ye S, Jiang H, Chen Q, Liu F et al (2009) Arabidopsis ASA1 is important for jasmonate-mediated regulation of auxin biosynthesis and transport during lateral root formation. Plant Cell 21:1495–1511. doi:10.1105/tpc.108.064303
Teaster ND, Motes CM, Tang Y, Wiant WC, Cotter MQ, Wang YS, Kilaru A, Venables BJ, Hasenstein KH, González G (2007) N-acylethanolamine metabolism interacts with abscisic acid signalling in Arabidopsis thaliana seedlings. Plant Cell 19:2454–2469. doi:10.1105/tpc.106.048702
Tiryaki I, Staswick PE (2002) An Arabidopsis mutant defective in jasmonate response is allelic to the auxin-signaling mutant axr1. Plant Physiol 130:887–894. doi:10.1104/pp.005272
Valencia-Cantero E, Flores-Cortez I, Ambriz-Parra J, Lopez-Albarran P, Velázquez-Becerra C (2015) Arthrobacter agilis UMCV2 accelerates growth of Pinus devoniana. Phyton-Int J Exp Bot 84:64–69
Van der Ent S, Van Wees SC, Pieterse CM (2009) Jasmonate signaling in plant interactions with resistance-inducing beneficial microbes. Phytochemistry 70:1581–1588. doi:10.1016/j.phytochem.2009.06.009
Velázquez-Becerra C, Macías-Rodríguez LI, López-Bucio J, Altamirano-Hernández J, Flores-Cortez I, Valencia-Cantero E (2011) A volatile organic compound analysis from Arthrobacter agilis identifies dimethylhexadecylamine, an amino-containing lipid modulating bacterial growth and Medicago sativa morphogenesis in vitro. Plant Soil 339:329–340. doi:10.1007/s11104-010-0583-z
Velázquez-Becerra C, Macías-Rodríguez LI, López-Bucio J, FloresCortez I, Santoyo G, Hernández-Soberano C, Valencia-Cantero E (2013) The rhizobacterium Arthrobacter agilis produces dimethylhexadecylamine, a compound that inhibits growth of phytopathogenic fungi in vitro. Protoplasma 250:1251–1262. doi:10.1007/s00709-013-0506-y
Von Rad U, Klein I, Dobrev PI, Kottova J, Zazimalova E, Fekete A, Hartmann A, Schmitt-Kopplin P, Durner J (2008) Response of Arabidopsis thaliana to N-hexanoyl-DL-homoserine-lactone, a bacterial quorum sensing molecule produced in the rhizosphere. Planta 229:73–85
Wasternack C (2007) Jasmonates. An update on biosynthesis, signal transduction and action in plant stress response, growth and development. Ann Bot London 100:681–697. doi:10.1093/aob/mct067
Wilmoth JC, Wang S, Tiwari SB, Joshi AD, Hage G, Guilfoyle TJ, Alonso JM, Ecker JR, Reed JW (2005) NPH4/ARF7 and ARF19 promote leaf expansion and auxin-induced lateral root formation. Plant J 43:118–130. doi:10.1111/j.1365-313X.2005.02432.x
Zhao Q, Zhang C, Jia Z, Huang Y, Li H, Song S (2015) Involvement of calmodulin in regulation of primary root elongation by N-3-oxo-hexanoyl homoserine lactone in Arabidopsis thaliana. Front Plant Sci 5:807. doi:10.3389/fpls.2014.00807
Acknowledgments
We thank the Coordinación de la Investigación Científica UMSNH (México) for funding this work via projects 2.22 (EVC) and 2.26 (JLB).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Additional information
Handling Editor: Peter Nick
Electronic supplementary materials
Below is the link to the electronic supplementary material.
Fig. S1
DMAs regulate Arabidopsis shoot and root development. Arabidopsis WT (Col-0) seedlings were germinated and grown on 0.2× MS agar medium for 10 days with or without different concentrations of DMAs. Photographs of representative wild-type (Col-0) seedlings are presented. Note that C12 and C14 were the most active DMAs on Arabidopsis plant development. The experiment was repeated twice with similar results. Scale bar = 1 cm (TIF 4949 kb)
Fig. S2
Selection of homozygous coi1 mutants from a heterozygous coi1/COI1 population. a Primary root length of WT (Col-0) seedlings and coi1 mutants in medium supplied with 4 μM JA. b Representative photograph showing the growth of coi1/COI1 seedlings on an agar plate supplemented with 4 μM JA. Notice the segregating plants with long primary roots corresponding to homozygous coi1 mutants (white arrows). Errors bar in (a) represent SE from 100 seedlings. Different letters indicate statistical differences at P < 0.05 (TIF 2516 kb)
Fig. S3
Shoot phenotype of WT and coi1 mutants in response to C16-DMA. a, b Representative photographs showing the growth of WT and coi1 seedlings grown side by side on agar plates supplied with the solvent (control) or 30 μM C16-DMA. Notice the strong growth-repressing effects of the compound on WT shoots but not in coi1 shoots (c, d). The experiment included 5 replicated plates per treatment. Scale bar= 1 cm (a, b) and 5 mm (c, d) (TIF 5837 kb)
Fig. S4
Effect of C16-DMA on root development of WT and myc2 seedlings. Arabidopsis wild-type (Col-0) and myc2 seedlings were germinated and grown side by side for 10 days on 0.2× MS medium supplied with varied C16-DMA concentrations. (a) Primary root length. b Lateral root number. c Representative plates showing the plant phenotypes. Errors bars represent SE from 20 seedlings. Different letters indicate statistical differences at P < 0.05. The experiment was repeated twice with similar results. Scale bar = 1 cm (TIF 3659 kb)
Rights and permissions
About this article
Cite this article
Raya-González, J., Velázquez-Becerra, C., Barrera-Ortiz, S. et al. N,N-dimethyl hexadecylamine and related amines regulate root morphogenesis via jasmonic acid signaling in Arabidopsis thaliana . Protoplasma 254, 1399–1410 (2017). https://doi.org/10.1007/s00709-016-1031-6
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00709-016-1031-6