Abstract
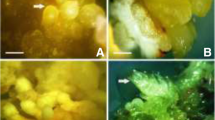
Podophyllum hexandrum Royle known as Indian mayapple is an important medicinal plant found only in higher altitudes (2,700 to 4,200 m) of the Himalayas. The highly valued anticancer drug Podophyllotoxin is obtained from the roots of this plant. Due to over exploitation, this endemic plant species is on the verge of extinction. In vitro culture for efficient regeneration and the production of podophyllotoxin is an important research priority for this plant. Hence, in the present study, an efficient plant regeneration system for mass multiplication through somatic embryogenesis was developed. We have screened P. hexandrum seeds collected from three different regions in the Himalayas to find their regenerative potentials. These variants showed variation in germination percentage as well as somatic embryogenic frequency. The seeds collected from the Milam area of Pithoragarh district showed better germination response (99.3 %) on Murashige and Skoog (MS) medium fortified with Gibberellic acid (GA3 [5 mg/l]) and higher direct somatic embryogenic frequency (89.6 %). Maximum production of embryogenic callus (1.2 g fresh weight [FW]) was obtained when cotyledons containing the direct somatic embryo clusters were cultured in MS medium supplemented with 2,4-dichlorophenoxyacetic acid (2,4-D [1.5 mg/l]) after 4 week of culture in complete darkness. In the present investigation, somatic embryogenesis was accomplished either by direct organogenesis or callus mediated pathways. The latter method resulted in a higher frequency of somatic embryo induction in hormone-free MS medium yielding 47.7 embryos/50 mg of embryogenic callus and subsequent germination in MS medium supplemented with GA3 (5 mg/l). Seventy-nine percent of embryos attained complete maturity and germinated into normal plants with well-developed roots. Systematic histological analysis revealed the origin of somatic embryo and their ontogenesis. The higher level of podophyllotoxin (1.8 mg/g dry weight [DW]) was recorded in germinated somatic embryos when compared to field grown plants. The present system can be widely used for mass propagation, transgenic recovery, and podophyllotoxin production for commercial utilization.



Similar content being viewed by others
Abbreviations
- 2,4-D:
-
2,4-dichlorophenoxy acetic acid
- ABA:
-
Abscisic acid
- DSE:
-
Direct somatic embryo
- GA3 :
-
Gibberellic acid
- HPLC:
-
High performance liquid chromatography
- IAA:
-
Indole-3-acetic acid
- IBA:
-
Indole-3-butyric acid
- MS:
-
Murashige and Skoog medium
- NAA:
-
Naphthaleneacetic acid
- PGR:
-
Plant growth regulators
- PTOX:
-
Podophyllotoxin
- RT:
-
Retention time
References
Ammirato PV (1974) The effects of abscisic acid on the development of somatic embryos from cells of caraway (Carum carvi L.). Bot Gaz 135:328–337
Anbazhagan VR, Ahn CH, Harada E, Kim YS, Choi YE (2008) Podophyllotoxin production via cell and adventitious root cultures of Podophyllum peltatum. In Vitro Cell Dev Biol Plant 44:494–501
Arumugam N, Bhojwani SS (1990) Somatic embryogenesis in tissue cultures of Podophyllum hexandrum. Can J Bot 68:487–491
Badhwar RL, Sharma BK (1963) A note on the germination of Podophyllum seeds. Indian For 89:445–447
Balzon TA, Luis ZG, Scherwinski-Pereira JE (2013) New approaches to improve the efficiency of somatic embryogenesis in oil palm (Elaeis guineensis Jacq.) from mature zygotic embryos. In Vitro Cell Dev Biol Plant 49:41–50
Bedir E, Khan I, Moraes RM (2001) Bioprospecting for podophyllotoxin. In: Janik J, Whipkey A (eds) Trends in new crops and new uses. ASHS, Alexandria, pp 545–549
Berlin GP, Miksche JP (1976) Botanical microtechnique and cytochemistry, 3rd edn. Iowa State University Press, Ames, Iowa, USA
Bhatt A, Rawal RS, Dhar U (2005) Germination improvement in Swertia angustifolia: a high value medicinal plant of Himalaya. Curr Sci 89(6):1008–1011
Brown DC, Thorpe TA (1995) Crop improvement through tissue culture. World J Microbiol Biotechnol 11:409–415
Bush EJ, Jones DW (1995) Asymmetric total synthesis of (−)-podophyllotoxin. J Chem Soc Perkin Trans 1:151–155
Chandra B, Palni LMS, Nandi SK (2006) Propagation and conservation of Picrorhiza kurrooa Royle ex Benth.: an endangered Himalayan medicinal herb of high commercial value. Biodivers Conserv 15:2325–2338
Choi YE, Kim JW, Yoon ES (1999) High frequency of plant production via somatic embryogenesis from callus or cell suspension cultures in Eleutherococcus senticosus. Ann Bot 83:309–314
Chugh A, Khurana PJ (2002) Gene expression during somatic embryogensis—recent advances. Curr Science 83:715–730
De Kroon H, Whigham DF, Watson MA (1991) Developmental ecology of mayapple: effects of rhizome severing, fertilization and timing of shoot senescence. Funct Ecol 5:360–368
Eudes F, Acharya S, Laroche A, Selinger LB, Cheng KJ (2003) A novel method to induce direct somatic embryogenesis, secondary embryogenesis and regeneration of fertile green cereal plants. Plant Cell Tiss Org Cult 73:147–157
Feher A, Pasternak T, Dudits D (2003) Transition of somatic plant cells to an embryogenic state. Plant Cell Tiss Org Cult 74:201–228
Fehr A (2006) Why somatic plant cells start to form embryos? In Mujid A, Samaj J, eds., Somatic Embryogenesis., Plant Cell Monographs,Vol.2, Robinson DG, series ed., Springer-Verlag, Berlin Heidelberg, Germany, pp. 85–101
Fischer-Iglesias C, Sundberg B, Neuhaus G, Jones AM (2001) Auxin distribution and transport during embryonic pattern formation in wheat. Plant J 26:115–129
Fitch MM (1993) High frequency somatic embryogenesis and plant regeneration from papaya hypocotyls callus. Plant Cell Tiss Org Cult 32(2):205–212
Gaj MD (2001) Direct somatic embryogenesis as a rapid and efficient system for in vitro regeneration of Arabidopsis thaliana (L.) Heynh. Plant Cell Tiss Org Cult 64:39–46
Jiménez VM (2005) Involvement of plant hormones and plant growth regulators on in vitro somatic embryogenesis. Plant Growth Regul 47:91–110
Kanchanapoom K, Domyoas P (1999) The origin and development of embryoids in oil palm (Elaeis guineensis Jacq.) embryo culture. Sci Asia 25:193–200
Kim SW, Oh SC, Liu JR (2003) Control of direct and indirect somatic embryogenesis by exogenous growth regulators in immature zygotic embryo cultures of rose. Plant Cell Tissue Org Cult 74(1):61–66
Kim YS, Lim S, Choi YE, Anbazhagan VR (2007) High frequency plant regeneration via somatic embryogenesis in Podophyllum peltatum L., an important medicinal plant for source of anticancer drug. Curr Sci 92:662–666
Krishnaraj S, Vasil IK (1995) Somatic embryogenesis in herbaceous monocots. In: Thorpe TA (ed) In vitro embryogenesis in plants. Kluwer, Dordrecht, pp 417–471
Majumder A, Jha S (2009) Biotechnological approaches for the production of potential anticancer leads podophyllotoxin and paclitaxel: an overview. J Bio Sci 1:46–69
Merkle SA, Sotak RJ, Wiecko AT, Sommer HE (1990) Maturation and conversion of Liriodendron tulipifera somatic embryos. In Vitro Cell Dev Biol 26:1086–1093
Mikuła A, Tykarska T, Zielińska M, Kurać M, Rybczyński J (2004) Ultrastructural changes in zygotic embryos of Gentiana punctata L. during callus formation and somatic embryogenesis. Acta Biol Cracov Ser Bot 46:109–120
Moraes-Cerdeira RM, Dayan FE, Bedir E, Barrett H, Burandt C Jr, Canel C (2002) The lignans of Podophyllum. In: Rahman AU (ed) Studies in natural product chemistry, vol 26. Elsevier, New York, pp 149–182
Nadeem M, Palni LMS, Purohit AN, Pandey H, Nandi SK (2000) Propagation and conservation of Podophyllum hexandrum Royle: an important medicinal herb. Biol Conserv 92:121–129
Nishiwaki M, Fujino K, Koda Y, Masuda K, Kikuta Y (2000) Somatic embryogenesis induced by the simple application of abscisic acid to carrot (Daucus carota L.) seedlings in culture. Planta 211:756–759
Orozco-Segovia A, Batis AI, Rojas-Aréchiga M, Mendoza A (2003) Seed biology of palms: a review. Palms 47:79–94
Paek KY, Chakrabarty D, Hahn EJ (2005) Application of bioreactor systems for large scale production of horticultural and medicinal plants. In: Hvoslef-Eide AK, Preil W (eds) Liquid culture systems for in vitro plant propagation. Springer, The Netherlands, pp 95–116
Pandey H, Nandi SK, Kumar A, Palni UT, Palni LMS (2007) Podophyllotoxin content in Podophyllum hexandrum Royle plants of known age of seed origin and grown at a lower altitude. Acta Physiol Plant 29:121–126
Pasternak T, Prinsen E, Ayaydin F, Miskolczi P, Potters G, Asard H, Van Onckelen H, Dudits D, Fehér A (2002) The role of auxin, pH and stress in the activation of embryogenic cell division in leaf protoplast-derived cells of alfalfa (Medicago sativa L.). Plant Physiol 129:1807–1819
Sadowska A, Wiweger M, Lata B, Obidoska G (1997) In vitro propagation of Podophyllum peltatum L. by the cultures of embrya and divided embrya. Biol Plant 39:331–336
Sajc L, Grubisic D, Vunjak-Novakovic G (2000) Bioreactors for plant engineering: an outlook for further research. Biochem Eng J 4:89–99
Sakakibara H, Takei K, Hirose N (2006) Interactions between nitrogen and cytokinin in the regulation of metabolism and development. Trends Plant Sci 11(9):440–448
Samant SS, Dhar U, Palni LMS (1998) Medicinal plants of Himalaya: diversity distribution and potential values. Himvikas, Gyanodaya Prakashan, Nainital
Scherwinski-Pereira JE, Guedes RS, Silva RA, Fermino PCP, Luis ZG, Freitas EO (2012) Somatic embryogenesis and plant regeneration in açaí palm (Euterpe oleracea). Plant Cell Tiss Org Cult 109:501–508
Sivanandhan G, Mariashibu TS, Arun M, Rajesh M, Kasthurirengan S, Selvaraj N, Ganapathi A (2011) The effect of polyamines on the efficiency of multiplication and rooting of Withania somnifera (L.) Dunal and content of some withanolides in obtained plants. Acta Physiol Plant 33:2279–2288
Sivanandhan G, Arun M, Mayavan S, Rajesh M, Jeyaraj M, Kapil Dev G, Manickavasagam M, Selvaraj N, Ganapathi A (2012a) Optimization of elicitation conditions with methyl jasmonate and salicylic acid to improve the productivity of withanolides in the adventitious root culture of Withania somnifera (L.) Dunal. Appl Biochem Biotechnol 168:681–696
Sivanandhan G, Arun M, Mayavan S, Rajesh M, Mariashibu TS, Manickavasagam M, Selvaraj N, Ganapathi A (2012b) Chitosan enhances withanolides production in adventitious root cultures of Withania somnifera (L.) Dunal. Ind Crop Prod 37:124–129
Sultan P, Shawl AS, Ramteke PW, Jan A, Chisti N, Jabeen N, Shabir S (2006) In vitro propagation for mass multiplication of Podophyllum hexandrum: a high value medicinal herb. Asian J Plant Sci 5:179–184
van Uden W, Pras N, Visser JF, Malingre TM (1989) Detection and identification of podophyllotoxin produced by cell cultures derived from Podophyllum hexandrum Royle. Plant Cell Rep 8:165–168
Vasil IK (2008) A short history of plant biotechnology. Phytochem Rev 7:387–394
Williams EG, Maheswaran G (1986) Somatic embryogenesis: factors influencing coordinated behaviour of cells as an embryogenic group. Ann Bot 57:443–462
Acknowledgments
The authors are thankful to the Life Science Research Board, Defence Research and Development Organisation (DRDO), Govt. of India for financial support (DLS/81/48222/LSRB–171 BTB/2008) used to carry out the present work. The corresponding author is thankful to the University Grants Commission (UGC), Govt. of India, for providing an emeritus fellowship under the BSR scheme. The first author is thankful to Prof. S.K. Nandi, G.B. Pant Institute of Himalayan Environment and Development, Almora, Uttarakhand, India, Prof. M.C. Nautiyal, H.N.B. Garhwal University, Garhwal Srinagar, Uttarakhand, India, and Dr. Lokho Puni IFS, Forest Research Institute, Dehradun, Uttaranchal for help with the collection of seeds at different locations. The first author is thankful to Dr. V. Nandhagopal, National College, Trichy, for his critical guidance in histology work. The authors are thankful to Dr. R. Boopathy, Head of the Department and Dr. S. Girija, Associate Professor, Department of Biotechnology, Bharathiar University, Coimbatore for providing HPLC facility.
Conflict of interest
The authors declare that they have no conflict of interest.
Author information
Authors and Affiliations
Corresponding author
Additional information
Handling Editor: Peter Nick
Rights and permissions
About this article
Cite this article
Rajesh, M., Sivanandhan, G., Jeyaraj, M. et al. An efficient in vitro system for somatic embryogenesis and podophyllotoxin production in Podophyllum hexandrum Royle. Protoplasma 251, 1231–1243 (2014). https://doi.org/10.1007/s00709-014-0632-1
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00709-014-0632-1