Abstract
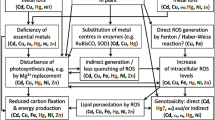
Interactive effects of two heavy metal pollutants Cd and Pb in the growth medium were examined on their uptake, production of reactive oxygen species (ROS), induction of oxidative stress and antioxidative defence responses in Indica rice (Oryza sativa L.) seedlings. When rice seedlings in sand culture were exposed to 150 μM Cd (NO3)2 or 600 μM Pb (CH3COO)2 individually or in combination for 8–16 days, a significant reduction in root/shoot length, fresh weight, relative water content, photosynthetic pigments and increased production of ROS (O2˙− and H2O2) was observed. Both Cd and Pb were readily taken up by rice roots and localisation of absorbed metals was greater in roots than in shoots. When present together in the growth medium, uptake of both the metals Cd and Pb declined by 25–40 %. Scanning electron microscope (SEM) imaging of leaf stomata revealed that Pb caused more distortion in the shape of guard cells than Cd. Dithizone staining of roots showed localisation of absorbed Cd on root hairs and epidermal cells. Both Cd and Pb caused increased lipid peroxidation, protein carbonylation, decline in protein thiol and increase in non-protein thiol. The level of reduced forms of non-enzymic antioxidants glutathione (GSH) and ascorbate (AsA) and their redox ratios (GSH/AsA) declined, whereas the activities of antioxidative enzymes superoxide dismutase (SOD) and guaiacol peroxidase (GPX) increased in metal treated seedlings compared to controls. In-gel activity staining also revealed increased intensities of SOD and GPX isoforms with metal treatments. Catalase (CAT) activity increased during early days (8 days) of metal exposure and declined by 16 days. Results suggest that oxidative stress is an important component in expression of Cd and Pb toxicities in rice, though uptake of both metals gets reduced considerably when present together in the medium.











Similar content being viewed by others
References
Aidid SB, Okamoto H (1993) Responses of elongation, growth rate, turgor pressure and cell wall extensibility of stem cells of Impatiens balsamina to lead, cadmium and zinc. Biometals 6:245–249
Ali MB, Vajpayee P, Tripathi RD, Rai UN, Singh SN, Singh PS (2003) Phytoremediation of lead, nickel, and copper by Salix acmophylla Boiss: role of antioxidant enzymes and antioxidant substances. Bull Environ Contam Toxicol 70:462–469
Ali B, Hasan SA, Hayat S, Hayat Q, Yadav S, Fariduddin Q, Ahmad A (2008) A role for brassinosteroids in the amelioration of aluminium stress through antioxidant system in mung bean (Vigna radiata L. Wilczek). Environ Exp Bot 62:153–159
Allen SE, Grimshaw HM, Rowland AP (1986) Chemical analysis. In: Mooren PD, Chapman SB (eds) Methods in plant ecology. Blackwell, Oxford, pp 285–344
Apel K, Hirt H (2004) Reactive oxygen species: metabolism, oxidative stress, and signal transduction. Annu Rev Plant Biol 55:373–39
Barcelo J, Vazquez MD, Poschenrieder C (1988) Structural and ultrastructural disorders in cadmium-treated bush bean plants (Phaseolus vulgaris L.). New Phytol 108:37–49
Beauchamp CO, Fridovich I (1971) Superoxide dismutase: improved assay and an assay applicable to acrylamide gels. Anal Biochem 44:276–287
Beers RF, Sizer IW (1952) Colorimetric method for estimation of catalase. J Biol Chem 195:133–139
Bradford MM (1976) A rapid and sensitive method for the quantification of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem 72:248–254
Bressler JP, Olivi L, Cheong JH, Kim Y, Bannon D (2004) Divalent metal transporter 1 in lead and cadmium transport. Ann N Y Acad Sci 1012:142–152
Cho U, Seo NH (2005) Oxidative stress in Arabidopsis thaliana exposed to cadmium is due to hydrogen peroxide accumulation. Plant Sci 168:113–120
Clemens S (2006) Toxic metal accumulation, response to exposure and mechanism of tolerance in plants. Biochimie 88:1707–1719
Davis BJ (1964) Disc electrophoresis. II. Method and application to human serum proteins. Ann NY Acad Sci 121:404–427
de Kok LJ, Kuiper PJC (1986) Effect of short-term dark incubation with chloride and selenate on the glutathione content of spinach leaf discs. Physiol Plant 68:477–482
Deng X, Xia Y, Hu W, Zhang H, Shen Z (2010) Cadmium-induced oxidative damage and protective effects of N-acetyl-l-cysteine against cadmium toxicity in Solanum nigrum L. J Hazard Mater 180:722–729
Dubey RS (2010) Metal toxicity, oxidative stress and antioxidative defense system in plants. In: Gupta SD (ed) Reactive oxygen species and antioxidants in higher plants. Science Publishers, CRC Press, Taylor and Francis Group, USA, pp 177–203
Egley GH, Paul RN, Vaughn KC, Duke SO (1983) Role of peroxidase in the development of water impermeable seed coats in Sidaspinosa L. Planta 157:224–232
Fecht-Christoffers MM, Maier P, Horst WJ (2003) Apoplastic peroxidase and ascorbate are involved in manganese toxicity and tolerance of Vigna unguiculata. Physiol Plant 117:237–244
Frahry G, Schopfer P (2001) NADH-stimulated, cyanide-resistant superoxide production in maize coleoptiles analysed with a tetrazolium-based assay. Planta 212:175–183
Garg N, Manchanda G (2009) ROS generation in plants: boon or bane? Plant Biosys 143:8–96
Gonnelli C, Galardi F, Gabbrielli R (2001) Nickel and copper tolerance and toxicity in three tuscan populations of Silene paradoxa. Physiol Plant 113:507–514
Griffith OW (1980) Determination of glutathione disulphide using glutathione reductase and 2-vinylpyridine. Anal Biochem 6:207–212
Gupta DK, Huang HG, Yang XE, Razafindrabe BHN, Inouhe M (2010) The detoxification of lead in Sedum alfredii H. is not related to phytochelatins but the glutathione. J Hazard Mater 177:437–444
Hancock J, Desikan R, Harrison J, Brigh J, Hooley R, Neill S (2006) Doing the unexpected: proteins involved in hydrogen peroxide perception. J Exp Bot 57:1711–1728
Heath RL, Packer L (1968) Photoperoxidation in isolated chloroplasts. Kinetics and stoichiometry of fatty acid peroxidation. Arch Biochem Biophys 125:189–198
Hsu YT, Kao CH (2004) Cd toxicity is reduced by nitric oxide in rice leaves. Plant Growth Regul 42:227–238
Islam E, Liu D, Li T, Yang X, Jin X, Mahmood Q, Tian S, Li J (2008) Effect of Pb toxicity on leaf growth, physiology and ultrastructure in the two ecotypes of Elsholtzia argyi. J Hazard Mater 154:914–926
Jana S, Choudhuri MA (1981) Glycolate metabolism of three submerged aquatic angiosperms during aging. Aquat Bot 12:345–354
Kaur S, Kamli MR, Ali A (2011) Role of arsenic and its resistance in nature. Can J Microbiol 57:769–774
Kim SY, Lim JH, Park MR et al (2005) Enhanced antioxidant enzymes are associated with reduced hydrogen peroxide in barley roots under saline stress. J Biochem Mol Biol 38:218–224
Klapheck S, Fliegner W, Zimmer I (1994) Hydroxymethyl-phytochelatins [(gamma- glutamyl-cysteine) n,-Serine] are metal-induced peptides of the Poaceae. Plant Physiol 104:1325–1332
Kristiansen KA, Jensen PE, Møller IM, Schulz A (2009) Monitoring reactive oxygen species formation and localisation in living cells by use of the fluorescent probe CM-H2DCFDA and confocal laser microscopy. Physiol Plant 136:369–383
Kumar A, Prasad MNV, Sytar O (2012) Lead toxicity, defense strategies and associated indicative biomarkers in Talinum triangulare grown hydroponically. Chemosphere 89:1056–1065
Law MY, Charles SA, Halliwell B (1983) Glutathione and ascorbic acid and spinach (Spinaceaoleracea) chloroplasts: the effect of hydrogen peroxide and paraquat. Biochem J 210:899–903
Levine RL, Williams J, Stadtman ER, Shacter E (1994) Carbonyl assays for determination of oxidatively modified proteins. Methods Enzymol 233:346–357
Lichtenthaler HK (1987) Chlorophylls and carotenoids: pigments of photosynthetic biomembranes. Methods Enzymol 148:350–382
Maheshwari R, Dubey RS (2009) Nickel induced oxidative stress and the role of antioxidant defense in rice seedlings. Plant Growth Regul 59:37–49
Mishra S, Dubey RS (2005) Heavy metal toxicity induced alterations in photosynthetic metabolism in plants. In: Pessarakli M (ed) Handbook of Photosynthesis, 2nd edn. CRC Press, Taylor and Francis Group, New York, pp 845–863
Mishra HP, Fridovich I (1972) The role of superoxide anion in autooxidation of the epinephrine and sample assay for SOD. J Biol Chem 247:3170–3175
Mohanty S, Grimm B, Tripathy BC (2006) Light and dark modulation of chlorophyll biosynthetic genes in response to temperature. Planta 224:692–699
Møller IM, Jensen PE, Hansson A (2007) Oxidative modifications to cellular components in plants. Annu Rev Plant Biol 58:459–481
Pathan AK, Bond J, Gaskin RE (2008) Sample preparation for scanning electron microscopy of plant surfaces-Horses for courses. Micron 39:1049–1061
Pielichowska M, Wierzbicka M (2004) The uptake and localization of cadmium by Biscutella leavigata a cadmium hyper accumulator. Acta Biol Cracoviensia, Ser Botanika 46:57–63
Pompella A, Maellaro E, Casini AF, Ferrali M, Ciccoli L, Comporti M (1987) Measurement of lipid peroxidation in vivo: a comparison of different procedures. Lipids 22:206–211
Pourrut B, Shahid M, Dumat C, Winterton P, Pinelli E (2011) Lead uptake, toxicity and detoxification in plants. Rev Environ Contam Toxicol 213:113–136
Radotic K, Ducic T, Mutavdzic D (2000) Changes in peroxidase activity and isozymes in spruce needles after exposure to different concentrations of cadmium. Environ Exp Bot 44:105–113
Romero-Puertas MC, Palma JM, Gomez M, del Rio LA, Sandalio LM (2002) Cadmium causes the oxidative modification of proteins in pea plants. Plant Cell Environ 25:677–686
Romero-Puertas MC, Rodríguez-serrano M, Corpas FJ, Gómez M, Del Río LA, Sandalio LM (2004) Cadmium-induced subcellular accumulation of O2˙− and H2O2 in pea leaves. Plant Cell Environ 27:1122–1134
Sandalio LM, Rodriguez-Serrano M, del Rio LA, Romero-Puetas MC (2009) Reactive oxygen species and signalling in cadmium toxicity. In: Puppo A, del Rio LA (eds) Reactive Oxygen Species and Plant Signaling. Springer-Verlag, Berlin, pp 175–189
Schützendübel A, Schwanz P, Teichmann T, Gross K, Langenfeld-Heyser R, Godbold DL, Polle A (2001) Cadmium-induced changes in antioxidative systems, hydrogen peroxide content and differentiation in scot pine (Pinus sylvestris) roots. Plant Physiol 127:887–892
Shah K, Kumar RG, Verma S, Dubey RS (2001) Effect of cadmium on lipid peroxidation, superoxide anion generation and activities of antioxidant enzymes in growing rice seedlings. Plant Sci 161:1135–1144
Sharma P, Dubey RS (2007) Involvement of oxidative stress and role of antioxidative defense system in growing rice seedlings exposed to toxic concentrations of aluminum. Plant Cell Rep 26:2027–2038
Sharma P, Jha AB, Dubey RS, Pessarakli M (2012) Reactive oxygen species, oxidative damage, and antioxidative defense mechanism in plants under stressful conditions. J Bot. doi:10.1155/2012/217037
Sharma R, Vleesschauwera DD, Sharma MK, Ronald PC (2013) Recent advances in dissecting stress-regulatory cross talk in rice. Mol Plant 6:250–260
Srivastava S, Dubey RS (2011) Manganese-excess induces oxidative stress, lowers the pool of antioxidants and elevates activities of key antioxidative enzymes in rice seedlings. Plant Growth Regul 64:1–16
Srivastava RK, Dubey RS (2012) Metal toxicity, production of reactive oxygen species and their consequences in plants. In: Hemantaranjan A (ed) Advances in plant physiology, vol 13. Scientific Publishers, Jodhpur, pp 415–456
Thordal-Christensen H, Zhang Z, Wei Y, Collinge DB (1997) Subcellular localization of H2O2 in plants, H2O2 accumulation in papillae and hypersensitive response during barley powdery mildew interaction. Plant J 11:1187–1194
Tran TA, Popova LP (2013) Function and toxicity of cadmium in plants: recent advances and future prospects. Turkish J Bot 37:1–13
Ushimaru T, Kanematsu S, Shibasaka M, Tsuji H (1999) Effect of hypoxia on antioxidant enzymes in aerobically grown rice (Oryza sativa) seedlings. Physiol Plant 107:181–187
Verma S, Dubey RS (2003) Influence of lead toxicity on photosynthetic pigments, lipid peroxidation and activities of antioxidant enzymes in rice plants. Indian J Agril Biochem 16:17–22
Weatherley PE (1950) Studies in the water relation of cotton plant. I. The field measurement of water deficits in leaves. New Phytol 49:81–97
Yadav SK (2010) Heavy metals toxicity in plants: an overview on the role of glutathione and phytochelatins in heavy metal stress tolerance of plants. South African J Bot 76:167–179
Yoshida S, Forno DA, Cock JH, Gomez KA (1976) Laboratory manual for physiological studies of rice, 3rd edn. International Rice Research Institute, Manila, Philippines, p 83
Zhang LH, Li PJ, Li XM, Meng XL, Xu CB (2005) Effects of cadmium stress on the growth and physiological characteristics of wheat seedlings. Chin J Ecol 24:458–460
Zhang FQ, Zhang HX, Wang GP, Xu LL, Shen ZG (2009) Cadmium-induced accumulation of hydrogen peroxide in the leaf apoplast of Phaseolus aureus and Vicia sativa and the roles of different antioxidant enzymes. J Hazard Mater 168:76–84
Acknowledgments
RKS and PP are grateful to Banaras Hindu University for providing Research Fellowships to conduct this work. Authors gratefully acknowledge Advanced Instrumentation Research Facility (AIRF), Jawaharlal Nehru University, New Delhi, for providing SEM facility and more specially to Dr. Ruchita Pal instrument in-charge SEM facility for her esteemed support and cooperation during the analysis.
Conflict of interest
The authors declare that they have no conflict of interest.
Author information
Authors and Affiliations
Corresponding author
Additional information
Handling Editor: Bhumi Nath Tripathi
Rights and permissions
About this article
Cite this article
Srivastava, R.K., Pandey, P., Rajpoot, R. et al. Cadmium and lead interactive effects on oxidative stress and antioxidative responses in rice seedlings. Protoplasma 251, 1047–1065 (2014). https://doi.org/10.1007/s00709-014-0614-3
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00709-014-0614-3