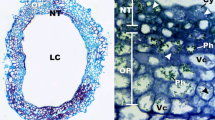
Abstract
Insect galls may present nutritive tissues with distinct cytological features related to the order of the gall inducer. Galling Lepidoptera larvae chew plant cells and induce the redifferentiation of parenchymatic cells into nutritive ones. The nutritive cells in the galls induced by a microlepidoptera on the leaves of Tibouchina pulchra (Cham.) Cogn. (Melastomataceae) are organelle-rich, with developed Golgi apparatus, endoplasmic reticulum, ribosomes, polyribosomes, mitochondria, plastids, and one great central or several fragmented vacuoles. The nonobservance of the nuclei in the nutritive cells deserves special attention, and confers a similarity between the nutritive cells and the vascular conductive ones. The great amount of rough endoplasmic reticulum, ribosomes, polyribosomes, and mitochondria is indicative of the high metabolic status of these cells. They are vascular cambium-like, with high protein synthesis and lipid storage. The proteins are essential to enzymatic metabolism, and secondarily, to larvae nutrition, similarly to the lipid droplets which confer energetic profile to these nutritive cells. The living enucleated cells receive mRNA from their neighbor ones, which may support the high metabolic profile of endoplasmic reticulum and ribosomes observed in galls. Thus, the nutritive cells are stimulated by the galling larvae activity, generating a new cell type, whose redifferentiation includes a mix of intrinsic and common plant pathways.


Similar content being viewed by others
References
Barnewall EC, De Clerck-Floate RA (2012) A preliminary histological investigation of gall induction in an unconventional galling system. Arthropod-Plant Interactions 6:449–459. doi:10.1007/s11829-012-9193-4
Begnani CN (2001) Cloroplastos. In: Carvalho HF, Recco-Pimentel SM (eds) A Célula. Malone, Campinas, pp 187–198
Begum S, Nakaba S, Oribe Y, Kubo T, Funada R (2010) Changes in the localization and levels of starch and lipids in cambium and phloem during cambial reactivation by artificial heating of main stems of Cryptomeria japonica trees. Ann Bot 106(6):885–895. doi:10.1093/aob/mcq185
Berlyn GP, Miksche JW (1976) Botanical microtechnique and cytochemistry. Iowa State, Ames
Bertachini-Labello C, Carvalho HF (2001) Retículo endoplasmático. In: Carvalho HF, Recco-Pimentel SM (eds) A Célula. Malone, Campinas, pp 127–137
Bertachini-Labello C, Dolder H, Carvalho HF (2001) Complexo de Golgi. In: Carvalho HF, Recco-Pimentel SM (eds) A Célula. Malone, Campinas, pp 138–148
Bronner R (1992) The role of nutritive cells in the nutrition of Cynipids and Cecidomyiids. In: Shorthouse JD, Rohfritsch O (eds) Biology of Insect Induced-galls. Oxford University Press, Oxford, pp 118–137
Carneiro MAA, Borges RAX, Araújo APA, Fernandes GW (2009) Insetos indutores de galhas da porção sul da Cadeia do Espinhaço, Minas Gerais, Brasil. Rev Bras Entomol 53(4):570–592. doi:10.1590/s0085-56262009000400007
Chessen G, Fahn A (1988) Cell hypertrophy in stems of Pinus halepensis infested by Matsucoccus josephi. Protoplasma 143(2–3):111–117
Doering-Saad C, Newbury HJ, Couldridge CE, Bale JS, Pritchard J (2006) A phloem-enriched cDNA library from Ricinus: insights into phloem function. J Exp Bot 57(12):3183–3193. doi:10.1093/jxb/erl082
Dreger-Jauffret F, Shorthouse JD (1992) Diversity of gall-inducing insects and their galls. In: Shorthouse JD, Rohfritsch O (eds) Biology of Insect Induced-galls. Oxford University Press, Oxford, pp 60–86
Evert RF (1977) Phloem structure and histochemistry. Ann Rev Pl Physiol 28:199–222
Evert RF, Bharati P, Deshpande P (1969) Electron microscope investigation of sieve-element ontogeny and structure in Ulmus americana. Protoplasma 68:403–432
Farrar JJ, Evert RF (1997) Seasonal changes in the ultrastructure of the vascular cambium of Robinia pseudoacacia. Trees 11:191–202
Gomes L, Pimentel ER, Recco-Pimentel SM (2001) Ribossomos e a Síntese protéica. In: Carvalho HF, Recco-Pimentel SM (eds) A Célula. Malone, Campinas, pp 116–126
Guellete BS, Benning UF, Hoffmann-Benning S (2012) Identification of lipids and lipid-binding proteins in phloem exudates from Arabidopsis thaliana. J Exp Bot 63(10):3603–3616. doi:10.1093/jxb/ers028
Heldt HW, Piechulla B (2011) Plant biochemistry. Academic, London
Isaias RMS, Oliveira DC, Carneiro RGS (2011) Role of Euphalerus ostreoides (Hemiptera: Psylloidea) in manipulating leaflet ontogenesis of Lonchocarpus muehlbergianus (Fabaceae). Bot 89:581–592. doi:10.1139/b11-048
Johansen DA (1940) Plant microtechnique. Mc-Graw-Hill Book, New York
Karnovsky MJ (1965) A formaldehyde-glutaraldehyde fixative of high osmolarity for use in electron microscopy. J Cell Biol 27:137–138
Kraus JE, Sousa HC, Resende MH, Castro NM, Vecchi C, Luque R (1998) Astra blue and basic fuchsin double staining of plant material. Biotechnic & Histohemistry 73:235–243
Lehninger AL, Nelson DL, Cox MM (1995) Princípios de Bioquímica. SARVIER, São Paulo
Lev-Yadun S (2003) Stem cells in plants are differentiated too. Current Opinion in Plant Biology 4:93–100
Lima ES, Magenta MAG, Kraus JE, Vecchi C, Martins SE (2000) Levantamento preliminar de galhas entomógenas ocorrentes em plantas da restinga de Bertioga (SP). Anais do V simpósio de ecossistemas brasileiros: Conservação 3:39–46
Maffei ME, Mithofer A, Boland W (2007) Before gene expression: early events in plant-insect interaction. Trends Plant Sci 12:310–316. doi:10.1016/j.tplants.2007.06.001
Mani MS (1964) Ecology of plant galls. Dr. W Junk Publishers, The Hague
Mani MS (1992) Introduction to cecidology. In: Shorthouse JD, Rohfritsch O (eds) Biology of Insect Induced-galls. Oxford University Press, Oxford, pp 3–7
Mendonça MS Jr (2007) Plant diversity and galling arthropod diversity searching for taxonomic patterns in an animal-plant interaction in the Neotropics. Bol Soc Argent Bot 42(3–4):347–357
Meyer J (1987) Plant galls and gall inducers. Gebrüder Bomtraeger, Berlin
Meyer J, Maresquele HJ (1983) Anatomie des Galles. Gebrüder Bomtrager, Berlin
Moura MZD, Soares GLG, Isaias RMS (2008) Species-especific changes in tissue morphogenesis induced by two arthropod leaf gallers in Lantana camara (Verbenaceae). Austr J Bot 56:153–160. doi:10.1071/BT07131
Occhioni P (1979) “Galhas”, “Cecídias” ou “Tumores vegetais” em plantas nativas da flora do Brasil. Leandra 8–9:5–35
Oliveira DC, Isaias RMS (2010) Cytological and histochemical gradients induced by a sucking insect in galls of Aspidosperma australe Arg. Muell (Apocynaceae). Plant Sci 178:350–358. doi:10.1016/j.plantsci.2010.02.002
Oliveira DC, Christiano JCS, Soares GLG, Isaias RMS (2006) Reações de defesas químicas e estruturais de Lonchocarpus mulherbergianus (Fabaceae) à ação do galhador Euphalerus ostreoides (Hemiptera: Psyllidae). Rev Bras Bot 29(4):657–667
Oliveira DC, Magalhães TA, Carneiro RGS, Alvim MN, Isaias RMS (2010) Do Cecidomyiidae galls of Aspidosperma spruceanum (Apocynaceae) fit the pre-established cytological and histochemical patterns? Protoplasma 242:81–93. doi:10.1007/s00709-010-0128-6
Oliveira DC, Carneiro RGS, Magalhães TA, Isaias RMS (2011a) Cytological and histochemical gradients on two Copaifera langsdorffii Desf. (Fabaceae)—Cecidomyiidae gall systems. Protoplasma 248:829–837. doi:10.1007/s00709-010-0258-x
Oliveira DC, Isaias RMS, Moreira ASFP, Magalhães TA, Lemos-Filho JP (2011b) Is the oxidative stress caused by Aspidosperma spp. galls capable of altering leaf photosynthesis? Plant Sci 180:489–495. doi:10.1016/j.plantsci.2010.11.005
Pearse AGE (1968) Histochemistry: theoretical and applied, 3rd edn., v 1. JA Churchill Ltd., London
Pimentel E (2001) Mitocôndrias. In: Carvalho HF, Recco-Pimentel SM (eds) A Célula. Malone, Campinas, pp 160–171
Reynolds ES (1963) The use of lead citrate at high pH as an electron-opaque stain in electron microscopy. J Cell Biol 17:208–212
Robbers JE, Speedie MK, Tyler VE (1996) Pharmacognosy and pharmacobiotechnology. Williams & Wilkins, Baltimore
Rohfritsch O (1978) Three-dimensional study of cell organelles in the nutritive tissue of a gall (Liposthenes glechomae L. on Glechoma hederacea L.). Protoplasma 95:297–307
Rohfritsch O (1992) Patterns in gall development. In: Shorthouse JD, Rohfritsch O (eds) Biology of Insect Induced-galls. Oxford University Press, Oxford, pp 60–86
Schrader J, Moyle R, Bhalerao R, Hertzberg M, Lundeberg J, Nilsson P, Bhalerao RP (2004) Cambial meristem dormancy in trees involves extensive remodelling of the transcriptome. Plant J 40:173–187. doi:10.1111/j.1365-313X.2004.02199.x
Sennerby-Forsse L (1986) Seasonal variation in the ultrastructure of the cambium in young stems of willow (Salix viminalis) in relation to phenology. Physiologia Plantarum 67:529–537
Shorthouse JD (1986) Significance of nutritive cells in insect galls. Proc Entomol Soc Washington 88:368–375
Spurr AR (1969) A low-viscosity epoxy resin embedding medium for electron microscopy. J Ultrastruct Res 26:31–43
Stone GN, Schonrögge K (2003) The adaptive significance of insect gall morphology. Trends Ecol Evol 18(10):512–522. doi:10.1016/S0169-5347(03)00247-7
Tavares JS (1919) As cecídias do Brasil que se criam nas plantas da família Melastomataceae. Brotéria: Série Zoológica 15:18–40
Tavares JS (1922) Cecidologia Brazileira. As restantes das Famílias. Brotéria: Série Zoológica 20:5–48
Van Bel AJE, Ehlers K, Knoblauch M (2002) Sieve elements caught in the act. Trends Plant Sci 7(3):126–132. doi:10.1016/S1360-1385(01)02225-7
Vecchi C (1999) Galha foliar em Tibouchina pulchra (Cham.) Cogn. (Melastomataceae) morfo-anatomia e ontogenia. Dissertation (Botânica). Universidade de São Paulo, São Paulo
Vidal BC, Mello MLS (1987) Biologia celular. Atheneu, São Paulo, p 347
Acknowledgments
The authors thank CAPES, CNPq, and FAPEMIG for financial support and R.G.S. Carneiro for language revision.
Conflict of interest
The authors declare no conflict of interest in the manuscript entitled “The redifferentiaton of nutritive cells of galls induced by Lepidoptera on T. pulchra (Cham.) Cogn. reveals predefined patterns on plant development.”
Author information
Authors and Affiliations
Corresponding author
Additional information
Handling Editor: Hanns H. Kassemeyer
Rights and permissions
About this article
Cite this article
Vecchi, C., Menezes, N.L., Oliveira, D.C. et al. The redifferentiation of nutritive cells in galls induced by Lepidoptera on Tibouchina pulchra (Cham.) Cogn. reveals predefined patterns of plant development. Protoplasma 250, 1363–1368 (2013). https://doi.org/10.1007/s00709-013-0519-6
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00709-013-0519-6