Abstract
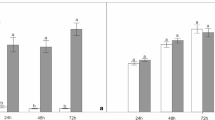
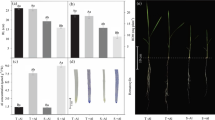
We have evaluated the impact of aluminum (Al) on germination, relative root growth, Al accumulation in roots tips, H2O2 levels, plasma membrane integrity, pigment levels, protein content, and the activities of superoxide dismutase (SOD) and catalase (CAT) in seedlings of the endangered Portuguese species Plantago algarbiensis and Plantago almogravensis. We found that up to 400 μM Al had no impact on the germination percentage in either species but inhibited root growth in a concentration-dependent manner (more severely in P. algarbiensis). Al accumulation in the root tips of both species was concentration dependent up to 200 μM but declined thereafter despite the absence of membrane damage. We observed a concentration-dependent induction of SOD activity but no change in CAT activity resulting in the accumulation of H2O2 (a known growth inhibitor), although its impact in P. almogravensis may be partially ameliorated by the accumulation of carotenoid pigments. Our data suggest an association between Al uptake, H2O2 production, and the inhibition of root growth during early seedling development in P. algarbiensis and P. almogravensis, although the latter is more tolerant towards higher concentrations of the metal.



Similar content being viewed by others
Abbreviations
- CAT:
-
Catalase
- FW:
-
Fresh weight
- MGT:
-
Mean germination time
- NBT:
-
Nitroblue tetrazolium
- ROS:
-
Reactive oxygen species
- SOD:
-
Superoxide dismutase
References
Aebi HE (1983) Catalase. In: Bergmeyer HU (ed) Methods of enzymatic analysis. Verlag Chemie, Weinhern, pp 273–286
Apel K, Hirt H (2004) Reactive oxygen species: metabolism, oxidative stress, and signal transduction. Annu Rev Plant Biol 55:373–399
Baker CJ, Mock NM (1994) An improved method for monitoring cell death in cell suspension and leaf disc assays using Evan’s blue. Plant Cell Tissue Organ Cult 39:7–12
Basu U, Good AG, Aung T, Slaski JJ, Basu A, Briggs KG, Taylor GJ (1999) A 23-kDa, root exudate polypeptide co-segregates with aluminum resistance in Triticum aestivum. Physiol Plant 106:53–61
Beauchamp CO, Fridovich I (1971) Superoxide dismutase: improved assays and assays applicable to acrylamide gels. Anal Biochem 44:276–287
Benavides MP, Gallego SM, Tomaro ML (2005) Cadmium toxicity in plants. Braz J Plant Physiol 17:21–34
Bewley DJ (1997) Seed germination and dormancy. Plant Cell 9:1055–1066
Bradford M (1976) A rapid and sensitive method for the quantification of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem 72:248–254
Branquinho C, Serrano HC, Pinto MJ, Martins-Loução MA (2007) Revisiting the plant hyperaccumulation criteria to rare plants and earth abundant elements. Environ Pollut 146:437–443
Buurman P, Jongmans AG (2005) Podzolisation and soil organic matter dynamics. Geoderma 125:71–83
Ellis RH, Roberts EH (1981) The quantification of ageing and survival in orthodox seeds. Seed Sci Technol 9:373–409
Ezaki B, Kiyohara H, Matsumoto H, Nakashima S (2007) Overexpression of an auxilin-like gene (F9E10.5) can suppress Al uptake in roots of Arabidopsis. J Exp Bot 58:497–506
Ezaki B, Nagao E, Yamamoto Y, Nakashima S, Enomoto T (2008) Wild plants, Andropogon virginicus L. and Miscanthus sinensis Anders, are tolerant to multiple stresses including aluminum, heavy metals and oxidative stresses. Plant Cell Rep 27:951–961
Gill SS, Tuteja N (2010) Reactive oxygen species and antioxidant machinery in abiotic stress tolerance in crop plants. Plant Physiol Biochem 48:909–930
Gonçalves S, Martins N, Romano A (2009) Micropropagation and conservation of endangered species Plantago algarbiensis and P. almogravensis. Biol Plant 53:774–778
Gui R, Leng H, Zhuang S, Zheng K, Fang W (2011) Aluminum tolerance in Moso Bamboo (Phyllostachys pubescens). Bot Rev 77:214–222
Halliwell B, Gutteridge JMC (1993) Free radicals in biology and medicine. Clarendon, Oxford
Kranner I, Colville L (2011) Metals and seeds: biochemical and molecular implications and their significance for seed germination. Environ Exp Bot 72:93–105
Labra M, Gianazza E, Waitt R, Eberini I, Sozzi A, Regondi S, Grassi F, Agradi E (2006) Zea mays L. protein changes in response to potassium dichromate treatments. Chemosphere 60:1234–1244
Lamhamdi M, Bakrim A, Aarab A, Lafont R, Sayah F (2011) Lead phytotoxicity on wheat (Triticum aestivum L.) seed germination and seedlings growth. R C Biologies 334:118–126
Larson RA (1988) The antioxidants of higher plants. Phytochem 27:969–978
Lefèvre I, Marchal G, Corréal E, Zanuzzi A, Lutts S (2009) Variation in response to heavy metals during vegetative growth in Dorycnium pentaphyllum Scop. Plant Growth Regul 59:1–11
Lichtenthaler HK (1987) Chlorophylls and carotenoids: pigments of photosynthetic biomembranes. Meth Enzymol 148:350–382
Loreto F, Velikova V (2001) Isoprene produced by leaves protects the photosynthetic apparatus against ozone damage, quences ozone products, and reduces lipid peroxidation of cellular membranes. Plant Physiol 127:781–787
Marciano DPRO, Ramos FT, Alvim MN, Magalhaes JR, França MGC (2010) Nitric oxide reduces the stress effects of aluminum on the process of germination and early root growth of rice. J Plant Nutr Soil Sci 173:885–891
Martins N, Gonçalves S, Palma T, Romano A (2011) The influence of low pH on in vitro growth and biochemical parameters of Plantago almogravensis and P. algarbiensis. Plant Cell Tissue Organ Cult 107:113–121
Martins N, Gonçalves S, Palma T, Romano A (2012) Seed germination of two critically endangered plantain species, Plantago algarbiensis and P. almogravensis (Plantaginaceae). Seed Sci Technol 40:144–149
Martins N, Gonçalves S, Andrade P, Valentão P, Romano A (2013a) Changes on organic acid secretion and accumulation in Plantago almogravensis Franco and P. algarbiensis Samp under aluminum stress. Plant Sci 198:1–6
Martins N, Gonçalves S, Romano A (2013b) Metabolism and aluminum accumulation in Plantago almogravensis and P. algarbiensis in response to low pH and aluminum stress. Biol Plant 57:325–331
Martins N, Osório ML, Gonçalves S, Osório J, Palma T, Romano A (2013c) Physiological responses of Plantago algarbiensis and P. almogravensis shoots and plantlets to low pH and aluminum stress. Acta Physiol Plant 35:615–625
Martins N, Osório ML, Gonçalves S, Osório J, Romano A (2013d) Differences in Al tolerance between Plantago algarbiensis and P. almogravensis reflect their ability to respond to oxidative stress. Biometals. doi:10.1007/s10534-013-9625-3
Nataraj M, Parmar S (2008) Biochemical response during the germination of raya and fenugreek seeds under heavy metal stress. J Cell Tissue Res 8:1589–1594
Pereira LB, Mazzanti CM, Gonçalves JF, Cargnelutti D, Tabaldi LA, Becker AG, Calgaroto NS, Farias JG, Battisti V, Bohrer D, Nicoloso FT, Morsch VM, Schetinger MR (2010) Aluminum-induced oxidative stress in cucumber. Plant Physiol Biochem 48:683–689
Pimentel NL, Wright VP, Azevedo TM (1996) Distinguishing early groundwater alteration effects from pedogenesis in ancient alluvial basins: examples from the Palaeogene of southern Portugal. Sediment Geol 105:1–10
Polle E, Konzak CF, Kittrick JA (1978) Visual detection of aluminum tolerance levels in wheat by hematoxylin staining of seedling roots. Crop Sci 18:823–827
Serrano HC, Pinto MJ, Martins-Loução MA, Branquinho C (2011) How does an Al-hyperaccumulator plant respond to a natural field gradient of soil phytoavailable Al? Sci Total Environ 409:3749–3756
Shaff JE, Schultz BA, Craft EJ, Clark RT, Kochian LV (2010) GEOCHEM-EZ: a chemical speciation program with greater power and flexibility. Plant Soil 330:207–214
Singh PK, Tewari RK (2003) Cadmium toxicity induced changes in plant water relations and oxidative metabolism of Brassica juncea L. plants. J Environ Biol 24:107–112
Tahara K, Yamanoshita T, Norisada M, Hasegawa I, Kashima H, Sasaki S, Kojima K (2008) Aluminum distribution and reactive oxygen species accumulation in root tips of two Melaleuca trees differing in aluminum resistance. Plant Soil 307:167–178
Verma S, Dubey RS (2003) Lead toxicity induces lipid peroxidation and alters the activities of antioxidant enzymes in growing rice plants. Plant Sci 164:645–655
Xu FJ, Jin CW, Liu WJ, Zang YS, Lin XY (2011) Pretreatment with H2O2 alleviates aluminum-induced oxidative stress in wheat seedlings. J Integr Plant Biol 53:44–53
Yadav SK, Mohanpuria P (2009) Responses of Camellia sinensis cultivars to Cu and Al stress. Biol Plant 53:737–740
Yamamoto Y, Kobayashi Y, Devi SR, Rikiishi S, Matsumoto H (2003) Oxidative stress triggered by aluminum in plant roots. Plant Soil 255:239–243
Zelinová V, Haluškova L, Huttová J, Illéš P, Mistrík I, Valentovičová K, Tamás L (2011) Short-term aluminium-induced changes in barley root tips. Protoplasma 248:523–530
Zhang H, Tan Z-Q, Hu L-Y, Wang S-H, Luo J-P, Jones RL (2010) Hydrogen sulfide alleviates aluminum toxicity in germinating wheat seedlings. J Integr Plant Biol 52:556–567
Acknowledgments
N. Martins and S. Gonçalves acknowledge grants SFRH/BD/48379/2008 and SFRH/BPD/31534/2006 from the Portuguese Science and Technology Foundation (FCT). This work was supported by the FCT project PTDC/AGR-AAM/ 102664/2008.
Conflicts of interest
The authors declare that they have no conflicts of interest.
Author information
Authors and Affiliations
Corresponding author
Additional information
Handling Editor: Néstor Carrillo
Rights and permissions
About this article
Cite this article
Martins, N., Gonçalves, S. & Romano, A. Aluminum inhibits root growth and induces hydrogen peroxide accumulation in Plantago algarbiensis and P. almogravensis seedlings. Protoplasma 250, 1295–1302 (2013). https://doi.org/10.1007/s00709-013-0511-1
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00709-013-0511-1