Abstract
Ascochyta pisi is a necrotrophic pathogenic fungus, which mainly survives between seasons through infected seeds. Defence responses of pea embryo axes to A. pisi were investigated in the heterotrophic phase of seed germination and during the transition from the heterotrophic to the autotrophic phase. Germinated pea seeds, both non-inoculated and inoculated with A. pisi, were cultured in perlite for 96 h. Polarographic studies performed on intact embryo axes of germinating pea seeds infected with A. pisi showed a high respiratory intensity in time from 48 to 96 h after inoculation. Forty-eight-hour embryo axes of germinating pea seeds exhibited the highest respiration rate, which in infected axes was maintained at the following time points after inoculation. Moreover, at 72 and 96 h after inoculation, respiratory intensity was by 64% and 73% higher than in the control. Electron paramagnetic resonance analysis revealed a higher concentration of semiquinone free radicals with g values of g || = 2.0031 ± 0.0004 and g ⊥ = 2.0048 ± 0.0004 in infected axes than in the control. Generation of superoxide anion radical was also higher in infected axes than in the control but stronger at 72 and 96 h after inoculation. Starting from 72 h after infection, the level of Mn2+ ions in infected axes decreased in relation to the control. At the same time, the highest activity of superoxide dismutase (EC 1.15.1.1) and catalase (EC 1.11.1.6) was observed in 72-h infected axes. In turn, the activity of peroxidase (EC 1.11.1.7) up to 72 h after infection was lower than in the control. In 48-h infected embryo axes, a very high level of pterocarpan pisatin was observed. Infection of germinating pea seeds with A. pisi restricted mainly the growth of the epicotyl, but did not inhibit the increase in length and fresh weight of root embryo axes versus cultivation time. These results indicate that in pea during the stages of seed germination and early seedling growth, protective mechanisms are induced in embryo axes against A. pisi.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Plants, unlike mammals, lack mobile defender cells or a somatic adaptive immune system. Instead, they rely on the innate immunity of each cell and on systemic signals emanating from infection sites. The recognition of a pathogen by plants occurs through the use of transmembrane pattern recognition receptors or, acting largely inside the cell, polymorphic nucleotide-binding leucine-rich-repeat protein products, encoded by most R genes (Jones and Dangle 2006). Activation may induce defensive reactions, which are the result of highly coordinated sequential changes at the cellular level. A higher respiration rate appears shortly after infection—certainly by the time visible symptoms appear—and it continues to rise during the multiplication and sporulation of the pathogen. Respiration increases because large amounts of energy are needed and used for a rapid production or mobilization of defence mechanisms in the cells. Moreover, several changes in the metabolism of the diseased plant accompany an increase in respiration after infection. The activity of several enzymes of the respiratory pathways seems to be higher. The accumulation and oxidation of phenolic compounds, many of which are associated with defence mechanisms in plants, are also greater during increased respiration (Agrios 1997). During the oxidation of phenols by peroxidase (POX) or polyphenol oxidase, semiquinone radicals are formed, which exhibit high reactivity and cytotoxicity (Kehrer 1993). As it was reported by O’Brien (1991), the toxicity of quinones is, in a large part, a consequence of reactive oxygen intermediates formed during redox cycling between oxidized quinones and reduced phenols. Semiquinone radicals readily donate electrons to molecular oxygen, forming superoxide anions (O2 •−) (Testa 1995) that are rapidly dismutated by superoxide dismutase (SOD) to form hydrogen peroxide (H2O2) (Mehdy 1994). The formation of quinones and free radicals can inactivate enzymes, which may be part of the arsenal of weapons used by the pathogen (Hammerschmidt 2005). In addition, these oxidized phenolic species exhibit an enhanced antimicrobial activity and thus may be directly involved in stopping pathogen development (Appel 1993). Electron paramagnetic resonance (EPR) spectroscopy may be applied to measure free radicals, including semiquinone radicals and paramagnetic metal ions in biological systems. For example, Zweier and Kuppusamy (1988) demonstrated that EPR spectroscopy may be applied to directly measure in vivo free radical metabolism and tissue oxygen consumption. In our earlier study, the direct detection of stable radicals in isolated pea embryo axes with different carbohydrate levels by EPR spectroscopy revealed postinfection accumulations of these radicals in the tissues. However, the intensity of the generation of these radicals in embryo axes, which were cut off from cotyledons and infected with pathogenic fungi by injection and cultured in vitro on a medium with sucrose or without it, varied markedly depending on the inoculum, Fusarium oxysporum or Ascochyta pisi (Morkunas et al. 2008).
Germination of pea seeds investigated in this study is hypogeic in character, i.e. cotyledons during the germination process are found in perlite and the epicotyl emerges above the perlite surface as the first between 72 and 96 h culture. Pea seeds in their reserve material pool contain over 60% starch and proteins, i.e. in the heterotrophic phase of germination, for metabolic processes using mainly carbon skeletons formed as a result of their hydrolytic degradation. Only at 96 h germination, when the epicotyl emerges above the surface of perlite, the seedling starts to independently synthesize carbohydrates. Thus, in this paper, we show mobilization of defence mechanisms in intact embryo axes of germinating pea seeds that have contact with cotyledons, against the pathogenic fungus A. pisi in the heterotrophic phase and during the transition from the heterotrophic to the autotrophic phase. This experimental design is similar to natural conditions under which seeds typically germinate.
Tivoli and Banniza (2007) showed that seeds were the main source of the introduction and then dissemination of various Ascochyta spp. in many countries worldwide. It is known that pea is not only attacked by A. pisi but also by other Ascochyta species, namely Ascochyta pinodes [the anamorph of Mycosphaerella pinodes (Berk. and Blox.) Vestergr.] and Phoma medicaginis var. pinodella (Jones) Boerema that occur singly or in combination and are sometimes referred to as the “Ascochyta complex” (Faris-Mokaiesh et al. 1996). These three fungi are mentioned as the cause of pea Ascochyta blights. A. pisi is known to produce the toxic metabolite ascochitine. A correlation was shown between the pathogenicity of A. pisi and the in vitro production of ascochitine (Lepoivre 1982). Marcinkowska et al. (1991) also reported ascochitine production by A. pisi, but not by A. pinodes or Ascochyta pinodella. Fungi responsible for Ascochyta blight may be considered as hemibiotrophs, characterized by an initial biotrophic phase that is followed by a necrotrophic phase (Spoel et al. 2007). However, phytotoxins characteristic of the necrotrophic pathogen were isolated from the germination fluid spores and were suggested to be of importance in early Ascochyta blight development (Höhl et al. 1991).
The aim of this study was to investigate the defence mechanisms against A. pisi induced in the early stages of pea seed germination. Therefore, we analysed postinfection changes in respiration, the generation of semiquinone radicals, superoxide anion radical and the activity of antioxidant enzymes, i.e. superoxide dismutase, catalase (CAT) and peroxidase, in embryo axes of pea germinating seeds. At the same time, postinfection changes in pterocarpan pisatin concentration were determined. Pisatin is one of low molecular weight toxic compounds, known to be synthesized de novo by plant tissues in response to microbial infection (Sweigard et al. 1986). Besides, the effect of A. pisi (which acts locally, not systemically) on growth of pea embryo axes was analysed.
Materials and methods
Plant material and growth conditions
Pisum sativum L. cv. Kwestor seeds of the S-elite class were used in the experiments. Seeds were surface-sterilized, immersed in sterile water and left in an incubator (25°C). After 6 h of imbibition, the seeds were transferred onto filter paper (in Petri dishes) and immersed in a small amount of water in order to support further absorption. After a subsequent 18 h, imbibed seeds were sown in pots (45 seeds per pot) containing sterilized perlite, inoculated with a spore suspension of A. pisi and allowed to germinate at 23°C at an irradiance of 130 μmol m−2 s−1 (Philips TLD 58W/84 fluorescent lamps) under a light regime of 12:12 h light/darkness. Non-inoculated germinating seeds were used as a control.
Samples for analyses were collected after 48, 72 and 96 h of culture, immediately frozen in liquid nitrogen to determine free radical contents using EPR, pisatin concentration as well as the activities of the antioxidant enzymes. Respiration activity measurements and superoxide anion detection were performed on live material at particular time points for all culture variants. Length and fresh weight measurements were taken, and disease symptoms were identified. The results of measurements for 20 embryo axes from every experimental variant at the time points were recorded, and the mean was calculated ± standard deviation. Samples for analyses were collected after 48 h, since the embryo axes penetrates the seed coats between 24 and 48 h of germination.
Preparation of spore suspension and inoculation
The A. pisi fungus was obtained from the Collection of the Department of Phytopathology, the Warsaw University of Life Sciences. A. pisi were incubated in the dark at 25°C on Petri dishes (diameter 9 cm) on the potato dextrose agar (PDA) medium (Difco, pH 5.5). Spore suspensions were prepared after 5 weeks of A. pisi growth by washing the mycelium with sterile water and shaking with glass pearls. The number of spores was determined using a Bürker hemocytometer chamber. Inoculation was performed by spraying 50 ml of the spore suspension at a concentration of 8 × 106 spores/1 ml onto 45 germinating seeds.
Measurement of embryo axis respiration
The respiratory intensity of intact embryo axes was determined using a Clark-type Digital Model 10 oxygen electrode (Rank Brothers, Cambridge, UK). At specific time points, embryo axes were removed from the culture tubes and transferred into an oxygen electrode incubation chamber of 7 ml, containing 4 ml of fully aerated and stirred Heller medium. The decrease in oxygen concentration was measured for 15 min. After the measurements, embryo axes were weighed to determine the respiration rate per gram of fresh weight (FW). Oxygen uptake rate was expressed as nanomoles per minute per gram FW.
Electron paramagnetic resonance
Samples of 1 g fresh weight of embryo axes were frozen in liquid nitrogen and lyophilized in a Jouan LP3 freeze dryer. The lyophilized material was transferred to EPR-type quartz tubes of 4 mm in diameter. Electron paramagnetic resonance measurements were performed with a Bruker ELEXSYS X-band spectrometer. The EPR spectra were recorded at room temperature as derivatives of microwave absorption. A magnetic field modulation of about 2 G and a microwave power of 2 mW were typically used for all experiments to avoid line saturation. EPR spectra of Mn2+ and free radicals were recorded in the magnetic field range of 3,000–3,650 G and with 2,048 data points. In order to determine the number of paramagnetic centres (free radicals and Mn2+ ions) in the samples, the spectra were double-integrated and compared with the intensity of the standard Al2O3/Cr3+ single crystal with a known spin concentration (Morkunas et al. 2003, 2004, 2008; Morkunas and Bednarski 2008; Bednarski et al. 2010). Before and after the first integration of the spectra, small background corrections were made to obtain a reliable absorption signal before the second integration. Double integration of the free radicals was made separately, and this value was subtracted from the value obtained for the full 3,000–3,650-G scan range integration. Since samples placed in quartz tubes were of equal volume, but of different weight, EPR intensity data were recalculated per 1 g of dry sample.
Determination of superoxide anion (O2 •−)
The superoxide anion was detected according to Morkunas and Bednarski (2008), using dihydroethidium (DHE), a reduced form of ethidium bromide, which is non-fluorescent. Once in the cell, it is oxidized to give a fluorescent dye that binds to nearby DNA. The production of the superoxide anion in embryo axes of germinating pea seeds was observed following the staining of embryo axes with 10 μM DHE in 100 μM CaCl2, at pH 4.75. Fluorescence was visualized under a Zeiss LSM 510 laser scanning confocal microscope (argon laser excitation 450–490 nm, emission 520 nm or more, filter set no. 9, magnified ×50), and embryo axes were photographed using an AxioCam digital camera (Zeiss). An argon laser (488 nm) was used for excitation, with emission at 565–615 nm following background subtraction. Additionally, the production of the superoxide anion by the pathogenic fungus A. pisi growing on PDA medium was observed following the staining with 10 μM DHE in 100 μM CaCl2 at pH 4.75. After washing, A. pisi growing on PDA medium were observed using a Zeiss LSM 510 laser scanning confocal microscope.
Enzymatic assays
Samples of pea embryo axes (1 g) each were homogenized in 5 ml of 50 mM phosphate buffer (pH 7.0), containing 0.5 M NaCl and 1% PVP at 4°C and centrifuged at 15,000×g for 15 min. The activity of SOD (EC 1.15.1.1) was assayed according to Beauchamp and Fridovich (1971) by measuring its ability to inhibit the photochemical reduction of NBT. Measurement description is given in a study by Morkunas and Bednarski (2008). The reaction was started by switching on the light (two 15-W fluorescent lamps placed 30 cm below the test tubes) and proceeded for 15 min. Samples without the enzymatic extract in the examined tests were selected so that the absorption difference between the blank and the examined samples was approximately 50%. The amount of the enzyme that caused the inhibition of NBT reduction by 50% was assumed as a unit of SOD activity. The activity of CAT (EC 1.11.1.6) was determined by measuring H2O2 consumption (Morkunas et al. 2008). The activity of the enzyme was expressed as units per 1 mg of protein. Peroxidase (EC 1.11.1.7) activity towards a phenolic substrate, pyrogallol, was measured according to Nakano and Asada (1981). This method is based upon the measured content of purpurogallin—a product of pyrogallol oxidation (Morkunas and Gmerek 2007). Protein was determined according to Bradford (1976), using bovine serum albumin as a standard.
Analysis of pisatin
Isolation of phenolic compounds
Plant tissue was homogenized in 80% methanol (20 ml g−1 FW) and sonicated for 3 min using a VirTis VirSonic 60 sonicator. The suspension was filtered through a Büchner funnel and concentrated under vacuum at 40°C. Samples of plant extracts for LC analyses were prepared from 0.5 g FW pea tissue. The samples were purified and concentrated by solid-phase extraction on cartridges containing a cation exchanger and RP C-18 silica gel (Alltech, Carnforth, England) used in tandem, according to the method of Stobiecki et al. (1997).
Liquid chromatography (LC/UV)
Quantitative analyses were performed using an L-7000 Merck Hitachi HPLC pump, equipped with an L-7450 diode array detector (Darmstadt, Germany) and a Superspher 100 RP-18 column (250×2 mm; Merck). The p hydroxybenzoic acid was added to each analysed sample as an internal standard at a final concentration of 125 mM (LC retention time and UV spectral data did not interfere with those of the studied compounds). Qualitative analysis and profiles of aromatic compounds performed on HPLC at wavelengths of 259 and 350 nm from the embryo axes of P. sativum L.cv. Kwestor, both non-inoculated and inoculated with A. pisi, showed the presence of isoflavones and aromatic compounds with an unidentified structure.
Liquid chromatography–mass spectrometry (LC/ESI/MS/MS)
Analyses of plant extract samples were performed with an Agilent RR 1200 SL system connected to a micrOToF-Q mass spectrometer model by Bruker Daltonics (Bremen, Germany). An LC Superspher 100 RP-18 column (250 × 2 mm; Merck) was used. During LC/UV analyses, elution was carried out with two solvent mixtures: A (95% acetonitrile, 4.5% H2O, 0.5% acetic acid; v/v/v) and B (95% H2O, 4.5% acetonitrile, 0.5% acetic acid; v/v/v). Elution steps were as follows: 0–5 min isocratic at 10% A, 5–40 min linear gradient from 10% to 30% of A, 40–48 min linear gradient up to 100% of A and 48–60 min isocratic at 100% of A. Free isoflavone and pterocarpan (pisatin) aglycone and/or their glucosides were identified by comparing their retention times and mass spectra with the data obtained from respective standards.
The micrOToF-Q mass spectrometer consisted of an ESI source operating at a voltage of ±4.5 kV, nebulization with nitrogen at 1.2 bar and dry gas flow of 8.0 l/min at a temperature of 220°C. The instrument was operated using the micrOTOF Control programme version 2.3, and data were analysed using the Bruker Data Analysis ver. 4 package. Targeted MS/MS experiments were performed using a collision energy ranging from 10 to 25 eV, depending on the molecular masses of compounds. The instrument operated at a resolution higher than 15,000 full width at half maximum.
Statistical analysis
All determinations were performed in three independent experiments. Data shown were means of triplicates for each treatment; standard deviation was calculated and its range is shown in figures. The analysis of variance (ANOVA) was applied, and results were compared in order to verify whether means from independent experiments within a given experimental variant are significant. Analysis of variance between treatment means was also carried out. Additionally, Student’s test was performed at a significance level of 0.05. The effect of one factor, i.e. the pathogenic fungus, was investigated in the experiments.
Results
Respiration intensity
Polarographic studies performed on intact embryo axes of germinating pea seeds infected with A. pisi showed a high respiratory intensity in time from 48 to 96 h after inoculation (Fig. 1). Forty-eight-hour embryo axes of germinating pea seeds exhibited the highest respiration rate, which in infected axes was maintained at the following time points after inoculation. Moreover, in infected axes, the respiration rate was higher than in the control (the differences in results were statistically significant as analysed by ANOVA). In 72- and 96-h infected axes, respiration intensity was by 64% and 73% higher than in the control. In contrast to infected axes, in the control intact pea embryo axes, the respiration rate dropped markedly during the culture. However, the highest respiration intensity in those axes was recorded at 48 h of culture.
The effect of A. pisi on uptake of O2 (nanomoles per minute per gram FW) by embryo axes of germinating pea seeds cultured in perlite for 96 h. No significant differences were found between means from experiments within a given experimental variant (ANOVA at the level of significance p > 0.05, differences are statistically non-significant). Significant differences were observed between applied experimental variants (control vs. infected)
Concentration of free radicals and Mn2+ ions
Typical EPR spectra recorded for the control and infected samples are presented in Fig. 2a. The free radical line with the highest amplitude in the middle of the spectra is nonsymmetrical (Fig. 2b) and has the same shape for each sample. This strongly suggests that the g factor is anisotropic. Measurements by EPR spectroscopy show that after infection of pea embryo axes with A. pisi, the concentration of free radicals was higher than in the control (Fig. 2c). Thus, in 48-h infected axes of germinating seeds, the level of free radicals was by 16% higher; at 72 h, it was by 18% and at 96 h, it was by 37% higher than in the control. These free radicals had the spectroscopic splitting coefficients values g || = 2.0031 ± 0.0004 and g⊥ = 2.0048 ± 0.0004, respectively. An analysis of changes in the concentration of free radicals with time, both in the control and the infected axes, showed a decrease in the amounts of these radicals in 72-h axes in relation to 48-h axes, followed by an increase in 96-h axes. However, the highest concentration of these radicals (2.6 × 10−15 spins g−1 DW) was recorded in 96-h infected embryo axes of germinating seeds.
At the same time, next to the measurement of free radicals by EPR, the presence of manganese ions (Mn2+) was observed in intact embryo axes of germinating pea seeds (Fig. 2d). In time between 48 and 96 h of culture, the concentration of Mn2+ ions decreased both in the infected and control axes. Apart from that, starting from 72 h after infection, a lower level of these ions was found in infected axes than in the control. ANOVA showed also that differences in the concentrations of Mn2+ ions and free radicals between non-infected embryo axes and those infected with A. pisi were significant.
Detection of superoxide anion (O2 •−)
Considerable differences in the O2 •− level were detected in pea embryo axes, non-inoculated and inoculated with A. pisi, starting from 72 h after infection (Fig. 3a). Relative release of the superoxide anion was investigated by staining embryo axes with the superoxide anion-specific indicator, dihydroethidium (DHE). The presence of O2 •− oxidizes DHE to ethidium, whereupon it emits fluorescence. The DHE-derived fluorescence began to appear in embryo axes from 48 h after inoculation with A. pisi. However, it should be stressed that a much higher fluorescence was recorded at 72 and 96 h after inoculation than at 48 h. In addition, within 96 h after inoculation, the surface anion generation was significantly higher than within 72 h. In addition, independently from the above, no or little generation of superoxide anion was observed in the pathogenic fungus A. pisi growing on PDA medium (Fig. 3b).
A. pisi induced superoxide anion (O2 •−) production in embryo axes of germinating pea seeds. One to six confocal images of embryo axes of germinating pea seeds inoculated A. pisi and controls (a). Superoxide anion (O2 •−) generation by pathogenic fungus A. pisi growing on potato dextrose agar medium (b). The production of superoxide anion radicals was visualized by staining with DHE as described in the “Materials and methods”
Activity of antioxidant enzymes
An analysis of changes in the activity of SOD, CAT and POX in intact embryo axes of germinating pea seeds, both the control and the infected ones in the time period from 48 to 96 h of culture, showed a considerable increase in the activity of these enzymes between 48 and 72 h, followed by a decrease in their activity between 72 and 96 h (Fig. 4). The highest SOD and CAT activity was recorded in 72-h infected embryo axes (Fig. 4a, b). Thus, SOD activity at this time point was approximately 88 U mg−1 protein, and it was by approximately 63% higher than that of 48-h infected axes, while CAT activity was 125 U mg−1 protein and it was by approximately 79% higher than that of 48-h axes. ANOVA showed that differences in the above results were significant. Moreover, SOD and CAT activities in infected embryo axes were higher than in the control at all the time points, except for SOD activity in 48-h infected axes. In turn, POX activity up to 72 h after infection was lower in infected axes than in the control, while further on post infection, i.e. at 96 h, a slightly higher activity of this enzyme was observed in relation to the control (Fig. 4c).
Concentration of pisatin
After infection of germinating pea seeds caused by A. pisi a very high pisatin level was observed in 48-h embryo axes. At the following time points after inoculation, a rapid decrease was recorded in the level of this pterocarpan, dropping to the detection threshold (Fig. 5). Pisatin concentration in 48-h embryo axes infected with A. pisi was many times higher than in the control. The significant differences in pisatin concentration were observed between applied experimental variants as analysed by ANOVA. In the control tissues, the level of pisatin in 48- and 72-h intact embryo axes of germinating pea seeds was around the detection threshold, while at 96 h, when the seedling emerges over the ground surface and independently synthesizes carbohydrates, an increase was observed in the level of this metabolite.
The effect of A. pisi on growth of germinating pea seeds
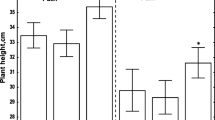
Infection of germinating pea seeds caused by a pathogenic fungus A. pisi resulted in an inhibition of embryo axis growth mainly at 48 h of culture. Roots in these axes 48 h after infection were by approximately 29% shorter than the control (Fig. 6a). At 48 h after infection, the strongest decrease was observed in fresh weight in comparison to the control, amounting to 47% (Fig. 6b). However, it needs to be stressed that between 48 and 72 h, and at 96 h after inoculation, a significant increase was observed in the length and fresh weight of root embryo axes vs. cultivation time; however, it was lower than in the control. The strong postinfection reduction of primary growth in germinating pea seeds included the epicotyl, since it was by approximately 1.5–2 times lower than that of the control axes at all the time points (Fig. 6c). Thus, epicotyl length in infected axes was by 38%, 36% and 48% lower than in the control. ANOVA confirmed that differences in epicotyl length between inoculated embryo axes and the control were significant.
Discussion
The results presented in this study show how pea embryo axes coordinate their defence against a necrotrophic fungus A. pisi during the early phase of seed germination and seedling development. Plants respond to the attack of fungal pathogens by activating different pathways. After seed infection, the high level of respiration intensity, observed in this study by means of polarography, much higher than in the control and maintained over all the investigated time points (Fig. 1), is probably connected first of all with the generation of energy required for the synthesis of defence compounds. This may be inferred from the fact that at an early stage of infection, i.e. at 48 h, when the embryo axes of the germinating seeds had just penetrated the seed coats, a very high level of pisatin, an antifungal substance of isoflavonoid origin, was recorded (Fig. 5). At the next time point, a very strong decrease was observed for this pterocarpan, which may suggest that pisatin could have been metabolized by the fungus A. pisi alone or its synthesis could have been stopped.
The biological activity of isoflavonoids in the host cells in response to infections may be connected with the inhibition of mycelium growth, elongation of conidiophores and spore germination. These processes are accompanied by various cytological phenomena, observed under light and electron microscopes, such as a rapid cessation of cytoplasm movement, its granulation, disorganisation of cellular organelles or damage to the plasmolemma (Skipp et al. 1977; Van Etten and Bateman 1971).
Agrios (1997) also reported that the increased respiration of diseased plants may be explained as a result of increased metabolism. In many plant diseases, growth is at first stimulated, protoplasmic streaming increases and materials are synthesized, translocated and accumulated in the diseased area. The energy required for these activities is derived from ATP produced through respiration. Secondly, the generation of reactive oxygen species (ROS) is an inherent element of aerobic metabolism, i.e. the high respiration rate recorded in this study may indicate an enhanced generation of reactive oxygen species (Fig. 3a), which in the embryo axis–A. pisi interaction may exhibit a toxic action towards the pathogen. The level of superoxide anion radicals at 48 h was low because these can interact with other radicals or endogenous substrates in the cell. In turn, their strong generation was observed at later times after inoculation. However, we need to remember here that necrotrophs themselves may use oxidation processes in the invasion of host cells (Gönner and Schlösser 1993). Thus, the generation of ROS may be both an element of the defence response of the plant and of an offensive strategy of the pathogen (Fig. 3). However, as it was noted in vitro in this study, no or little generation of the superoxide anion was observed in the pathogenic fungus A. pisi growing on PDA medium (Fig. 3b).
Moreover, as it was reported by Partridge (2003), after infection, the respiration of the pathogenic fungus should also be included in the results of total respiration of the host. In turn, Weir (2005) reported that when comparing the respiration of 1 g of Verticillium albo-atrum mycelium with 1 g fresh weight infected tomato stem tissue, it was concluded that the respiration of the pathogen in situ does not contribute markedly to the respiratory increase. It was suggested that the increase in respiration has its origin in the metabolism of the host, possibly due to stimulation by a fungal respiratory toxin, but not due to the augmentative effect of the pathogen’s respiration. Necrotrophs such as A. pisi depend on dead host tissues for nutrients and reproduction. These pathogens often secrete also enzymes and toxins that degrade and kill host cells to make nutrients available (Hancock and Huisman 1981; Glazebrook 2005). Besides, environmental conditions play also an important role in the development and dissemination of diseases caused by necrotrophic pathogens. The physiological plant growth stage, the form of the inoculum and inoculum concentration all affect the degree of infection. Thus, the seed germination stage studied here, comprising both a heterotrophic and autotrophic phase, is particularly significant to the ontogenetic development of plants because changes in the level of soluble carbohydrates during this phase may affect the susceptibility of germinating seed embryo axes to infections caused by pathogenic fungi (Morkunas et al. 2008, 2010).
Moreover, Paulech and Haspelova-Horvatovicovfi (1986) reported that root respiration of diseased barley plants slightly increased shortly after the inoculation with a parasitic fungus Erysiphe graminis as compared with the healthy plants. In contrast, respiration was significantly reduced at the later phase of pathogenesis. The decrease of root respiration is not due to a shortage of the respiration substrate but to a functional deficiency of certain mitochondria in root cells. In turn, as a consequence of fungal infection on sugar beet roots, the respiration rate increases and accumulation of reducing sugars was reported by Mumford and Wyse (1976).
In this study, apart from the high respiration rate at all the time points after inoculation, a higher generation of semiquinone radicals by EPR spectroscopy (Fig. 2a, c) and superoxide anion radicals was detected under a confocal microscope (Fig. 3a) in embryo axes of germinating pea seeds infected with A. pisi in relation to the control. However, only in 48-h infected embryo axes it was observed that the elevated respiratory activity might stimulate the formation of pisatin and enhance the formation of semiquinone radicals (Figs. 1, 2 and 5). In turn, at 72 and 96 h postinfection, higher rates of respiration were recorded, as well as the generation of semiquinone radicals and superoxide anion radicals higher than in the control, but not elevated level of pisatin. An enhanced generation of these free radicals may be included in the defence strategy of these axes against the pathogenic fungus, A. pisi.
It results from the literature data that semiquinone radicals may both exhibit a toxic action against the pathogen and be incorporated in such polymers as lignins by binding with ROS. It was additionally observed that they play an equally important role as reactive oxygen species in plant disease pathology (Höhl et al. 1991; Pearce et al. 1997). Quinones are common secondary metabolites with important roles in energy production, host defence and electron transport (Thomson 1987). Quinones are widely used medically and their cytotoxic effect is well-documented (O’Brien 1991). While some toxicity results from the binding of quinones directly to nucleic acids, proteins, lipids or carbohydrates, more significant are those mechanisms related to reactive oxygen intermediates. Semiquinone intermediates result from univalent quinone reductions catalyzed by several cellular enzymes (Testa 1995). Semiquinones readily donate electrons to oxygen, thereby generating superoxide anions. The superoxide anions subsequently generate hydroxyl and hydroperoxyl-free radicals that inactivate enzymes, break DNA strands and cause membrane lipid peroxidation (Smith 1985). A number of studies have indicated that phenol-oxidizing enzymes, such as polyphenol oxidases or polyphenol–peroxidase–H2O2, are involved in the oxidation of polyphenols into quinones and lignification of plants during the microbial invasion (Mohammadi and Kazemi 2002). Van Gestelen et al. (1998) reported that during defence responses against pathogenic elicitation, a controlled expression of phenolic compounds, together with peroxidases, could lead to the synthesis of apoplastic quinones. Subsequently, the induction of ROS could result in electron reduction of quinones by the plasma membrane or apoplastic oxidase. The existence of plasma membrane oxidases capable of reducing quinones to their semiquinones supports a hypothesis on a plausible alternative system for the regulation of the plant oxidative burst. As it was reported by Barbehenn et al. (2003), the measurement of most free radicals is limited by their reactivity, their short lifetime and low steady-state levels in biological samples. However, upon oxidation, phenols are converted to relatively stable free radicals (semiquinone or phenoxyl radicals), which can be measured directly by EPR spectroscopy. EPR spectroscopy has important characteristics as a method for the direct detection of free radicals in chemically complex biological samples, since it is technically simple and it does not require the isolation or chemical characterization of organic oxidation products. The enhanced postinfection generation of semiquinone radicals was also recorded in earlier investigations conducted by Morkunas et al. (2004) on embryo axes of germinating yellow lupine seeds cultured on perlite; at 72 and 96 h after inoculation with F. oxysporum f. sp. lupini, the concentration of these radicals was 50% higher than in the control. In turn, the high level of semiquinone radicals maintained in embryo axes with a high sucrose level inoculated with A. pisi, as reported by Morkunas et al. (2008), may indicate that they activate a different defence strategy than that observed in embryo axes with a high sucrose level inoculated with F. oxysporum.
Moreover, in this study, the presence of paramagnetic manganese (Mn2+) ions was recorded by EPR in embryo axes of germinating pea seeds (Fig. 2d). Starting from 72 h after inoculation with A. pisi, a decrease in the level of manganese ions was found in infected tissues, possibly indicating the utilization of these in metabolic processes (e.g. in the synthesis of SOD), which does not preclude Mn2+ uptake by the necrotrophic fungus itself in its defence against reactive oxygen species generated by the host plant (Horsburgh et al. 2002). A similar trend was observed after inoculation of pea embryo axes with F. oxysporum, cultured on a medium without sucrose, as the level of Mn2+ at time points from 24 to 96 h after inoculation decreased and, starting from 48 h after inoculation, it was lower than in non-inoculated embryo axes cultured without sucrose (Morkunas et al. 2008).
A premise in the former line of inference may be connected with the fact that starting from 72 h in embryo axes of germinating pea seeds infected with A. pisi, the activity of SOD was found to be considerably higher than in the control (Fig. 4a). Additionally, analyses of changes in the activity of CAT showed a much higher activity of this enzyme in infected tissues than in the control (Fig. 4b). For this reason, the high activity of SOD and CAT may indicate an essential role of these enzymes in ROS scavenging and thus in the modification of the redox state in pea cells, as well as the high efficiency of the antioxidant system in embryo axes of germinating pea seeds infected with A. pisi. In turn, the activity of POX determined towards pyrogallol was lower than in the control up to 72 h after inoculation (Fig. 4c). A leading role in scavenging of hydrogen peroxide in axes infected with A. pisi seems to be played by CAT. Moreover, in the early stages of seed germination, strong temporal changes in antioxidant enzyme activity of the control and infected tissues were observed in this work. It should be emphasized that at this stage, intensive structural and metabolic changes are involved in embryo activation (Morkunas et al. 2004). At 48 h of germination, the embryo axe has just recently penetrated the seed coat; up to 72 h of germination, this is the heterotrophic stage of germination and at 96 h, the transition from the heterotrophic to the autotrophic phase occurs, i.e. the seedling begins to emerge over the perlite surface and starts to independently synthesize carbohydrates.
An increase in CAT and SOD activities in chickpea roots infected by F. oxysporum f.sp. ciceris was also reported by García-Limones et al. (2002), although such responses occurred earlier in the incompatible interactions as compared with the compatible ones. Earlier increases in CAT and SOD activities in roots may be associated with resistance to fusarium wilt in chickpea. In turn, Kumar et al. (2009) revealed that an increased activity of antioxidant enzymes, i.e. CAT, SOD, glutathione reductase and glutathione S-transferase, in roots minimizes the chances of oxidative burst (an excessive production of reactive oxygen species), and therefore, Fusarium verticillioides might be protected from the oxidative defence system during colonization. A decrease in antioxidant enzyme activities in plants first inoculated with F. verticillioides and at day 10 inoculated with Piriformospora indica was observed when compared with plants inoculated with F. verticillioides alone. These reduced antioxidant enzyme activities due to the presence of P. indica help the plant to overcome the disease load of F. verticillioides.
In this work, a lower POX activity than that in the control was reported up to 72 h after infection. Von Tiedemann (1997) reported that all Botrytis cinerea isolates tested suppressed plant POX activity as compared to non-inoculated leaves of Phaseolus vulgaris.
We need to particularly stress the fact that at an early stage of infection caused by A. pisi in 48-h embryo axes of germinating pea seeds, a very high concentration of pisatin, an antifungal substance of isoflavonoid origin, was found, followed by its concentration around the detection threshold at the successive time points after infection (Fig. 5). The high level of this metabolite in 48-h embryo axes of germinating seeds infected with A. pisi may indicate that this isoflavone is incorporated in the defence mechanism, triggered shortly after the penetration of the seed coat by the embryo axis.
Moreover, Smith and Cruickshank (1986) revealed that the initial rate of pisatin accumulation appears to be dependent on the pea pod and independent of any time delays associated with conidial germination and elicitor accumulation. However, the final pisatin concentration accumulated in the infection droplet was dependent on the dose of the elicitor, irrespective of the nature and timing of the elicitor treatment. In turn, Oku et al. (1976) reported that inoculation of pea leaves with a pathogenic fungus Erysiphe pisi did not induce pisatin biosynthesis until the infection was completed, unlike the non-pathogen E. graminis hordei, which induced pisatin generation to a detectable amount by 12 h after inoculation. These facts strongly suggest that the pathogenic fungus has an ability to suppress the first step of defence reaction, i.e. pisatin production, in order to avoid the penetration-inhibiting action of pisatin. Moreover, pisatin administrated at an early stage of infection significantly prevented the infection (formation of secondary hyphae) of E. pisi on pea leaves even at concentrations that had been proved non-inhibitory to this fungus. These results indicated that pisatin was an infection inhibitor rather than an antimicrobial substance. The terminal step of (+) pisatin biosynthesis in P. sativum L. is the methylation of phenol (+)6a-hydroxymaackiain. Extracts from pea seedlings perform this reaction using S-adenosylmethionine as the methyl donor (Sweigard et al. 1986). Wasmann and Van Etten (1996) revealed that isolates of the fungus Nectria haematococca pathogenic to pea are able to detoxify the phytoalexin pisatin via cytochrome P450-mediated demethylation. Additionally, the induction of pisatin biosynthesis by an insect elicitor, such as Bruchin B (3-hydroxypropionate ester of long-chain diols), was also reported by Cooper et al. (2005). The recognition of a biotic factor by plants and receptor activation may induce defensive reactions, which are the result of highly coordinated sequential changes at the cellular level, comprising, among other changes, the synthesis of isoflavonoids, including pisatin.
Our other studies showed a very high isoflavonoid content observed in embryo axes of germinating yellow lupine seeds infected with F. oxysporum SCHLECHT f. sp. lupini at 48 h after inoculation, i.e. shortly after the embryo axes penetrated the seed coat. At this time point in infected lupine tissues, the concentrations of genistein glucosides and that of a free aglycone, genistein, were substantially higher than in the controls (Morkunas et al. 2010).
The elimination of the pathogen is determined by the speed and efficiency of early defence responses initiated by the plant. In conclusion, the presented results suggest that in germinating seeds of pea cv. Kwestor, a high respiration rate is stimulated within 48 h of inoculation, shortly after the embryo axes penetrated the seed coat. At the same time, a very strong accumulation of pisatin enhances the defence of embryo axes of germinating pea seeds against A. pisi. In addition, elevated levels of semiquinone radicals generation (of organic origin) may support this defence because they may exhibit a toxic action against the pathogen or be incorporated in the polymer by binding with ROS. Therefore, the level of superoxide anion radical generation at 48 h was low, and their strong generation was observed at a later time after inoculation. At 72 h, a stimulation of the antioxidant system was observed, together with a reduction of Mn2+ ions a postinfection increase occurred in the activity of SOD. Moreover, a high postinfection CAT activity was recorded. Between 48 and 96 h post-inoculation, embryo axis roots were observed to grow, which may suggest that the defence mechanisms induced from the moment of seed coat penetration by the embryo axis were strong enough not to cause an inhibition of the increment in length or fresh weight of embryo axis roots, despite the fact that the infection reduced epicotyl growth. Infection did not cause marked changes in the growth of embryo axis roots, which may suggest that other biochemical mechanisms might be more important in the plant–pathogen interactions.
Abbreviations
- EPR:
-
Electron paramagnetic resonance
- CAT:
-
Catalase
- EDTA:
-
Ethylenediaminetetraacetic acid
- NBT:
-
Nitro blue tetrazolium
- POX:
-
Peroxidase
- PVP:
-
Polyvinylpyrrolidone
- SOD:
-
Superoxide dismutase
References
Agrios GN (1997) How plants defend themselves against pathogens. In: Smith MD (ed) Plant pathology. Academic, San Diego, pp 93–114
Appel HM (1993) Phenolics in ecological interactions: the importance of oxidation. J Chem Ecol 19:1521–1552
Barbehenn RV, Poopat U, Spencer B (2003) Semiquinone and ascorbyl radicals in the gut fluids of caterpillars measured with EPR spectrometry. Insect Biochem Mol Biol 33:125–130
Beauchamp CH, Fridovich I (1971) Superoxide dismutase: improved assays and an assay applicable to acrylamide gels. Anal Biochem 44:276–287
Bednarski W, Ostrowski A, Waplak S (2010) Low temperature short-range ordering caused by Mn2+ doping of Rb3H(SO4)2. J Phys-Condens Matter 22:225901
Bradford M (1976) A rapid and sensitive method for quantitation of microgram quantities of protein using the principle of protein-dye binding. Anal Biochem 72:248–254
Cooper LD, Doss RP, Price R, Peterson K, Oliver JE (2005) Application of Bruchin B to pea pods results in the up-regulation of CYP93C18, a putative isoflavone synthase gene, and an increase in the level of pisatin, an isoflavone phytoalexin. J Exp Bot 56:1229–1237
Faris-Mokaiesh S, Boccara M, Denis J-B, Derrien A, Spire D (1996) Differentiation of the ‘Ascochyta complex’ fungi of pea by biochemical and molecular markers. Curr Gen 29:182–190
García-Limones C, Hervás A, Navas-Cortés JA, Jiménez-Díaz RM, Tena M (2002) Induction of antioxidant enzyme system and other oxidative stress markers associated with compatible and incompatible interactions between chickpea (Cicer arietinum L.) and Fusarium oxysporum f. sp. ciceris. Physiol Mol Plant Pathol 61:325–337
Glazebrook J (2005) Contrasting mechanisms of defense against biotrophic and necrotrophic pathogens. Annu Rev Phytopathol 43:205–227
Gönner MV, Schlösser E (1993) Oxidative stress in interactions between Avena sativa L. and Drechslera spp. Physiol Mol Plant Pathol 42:221–234
Hammerschmidt R (2005) Phenols and plant–pathogen interactions: the saga continues. Physiol Mol Plant Pathol 66:77–78
Hancock JG, Huisman OC (1981) Nutrient movement in host–pathogen systems. Ann Rev Phytopathol 19:309–331
Höhl MM, Weidmann C, Höhl C, Barz W (1991) Isolation of solanapyrone A, B and C from culture filtrate and spore germination fluids of Ascochyta rabiei and aspect of phytotoxin action. J Phytopathol 132:193–206
Horsburgh MJ, Wharton SJ, Karavolos M, Foster SJ (2002) Manganese: elemental defence for a life with oxygen? Trends Microbiol 10:496–501
Jones JD, Dangle JL (2006) The plant immune system. Nature 444:323–329
Kehrer JP (1993) Free radicals as mediators of tissue injury and disease. Crit Rev Toxicol 23:21–48
Kumar M, Yadav V, Tuteja N, Johri AK (2009) Antioxidant enzyme activities in maize plants colonized with Piriformospora indica. Microbiol 155:780–790
Lepoivre P (1982) Sensitivity of pea cultivars to ascochitine and the possible role of the toxin in the pathogenicity of Ascochyta pisi (Lib.). J Phytopathol (Phytopathologische Zeitschrift) 103:25–34
Marcinkowska J, Klos B, Shcherbakova A (1991) Ascochitine production by fungi responsible for Ascochyta diseases of pea. J Phytopathol 131:253–258
Mehdy MC (1994) Active oxygen species in plant defense against pathogens. Plant Physiol 105:467–472
Mohammadi M, Kazemi H (2002) Changes in peroxidase and polyphenol oxidase activities in susceptible and resistant wheat heads inoculated with Fusarum graminearum and induced resistance. Plant Sci 162:491–498
Morkunas I, Bednarski W (2008) Fusarium oxysporum induced oxidative stress and antioxidative defenses of yellow lupine embryo axes with different level of sugars. J Plant Physiol 165(3):262–277
Morkunas I, Gmerek J (2007) The possible involvement of peroxidase in defense of yellow lupine embryo axes against Fusarium oxysporum. J Plant Physiol 164(2):185–194
Morkunas I, Garnczarska M, Bednarski W, Ratajczak W, Waplak S (2003) Metabolic and ultrastructural responses of lupine embryo axes to sugar starvation. J Plant Physiol 160:311–319
Morkunas I, Bednarski W, Kozłowska M (2004) Response of embryo axes of germinating seeds of yellow lupine to Fusarium oxysporum. Plant Physiol Biochem 42:493–499
Morkunas I, Bednarski W, Kopyra M (2008) Defense strategies of pea embryo axes with different levels of sucrose to Fusarium oxysporum and Ascochyta pisi. Physiol Mol Plant Pathol 72:167–178
Morkunas I, Stobiecki M, Marczak Ł, Stachowiak J, Narożna D, Remlein-Starosta D (2010) Changes in carbohydrate and isoflavonoid metabolism in yellow lupine in response to infection by Fusarium oxysporum during the stages of seed germination and early seedling growth. Physiol Mol Plant Pathol 75:46–55
Mumford DL, Wyse RE (1976) Effect of fungus infection on respiration and reducing sugar accumulation of sugarbeet roots and use of fungicides to reduce infection. Journal of the ASSBT 19:157–162
Nakano Y, Asada K (1981) Hydrogen peroxide is scavenged by ascorbate-specific peroxidase in spinach chloroplasts. Plant Cell Physiol 22:867–880
O’Brien PJ (1991) Molecular mechanisms of quinone cytotoxicity. Chem Biol Interac 80:1–41
Oku H, Shiraishi T, Ouchi S (1976) Effect of preliminary administration of pisatin to pea leaf tissues on the subsequent infection by Erysiphe pisi DC. Ann Phytopath Soc Japan 42:597–600
Partridge JE (2003) Pathogen effects on host systems. In: Introductory plant pathology. Department of Plant Pathology, University of Nebraska—Lincoln
Paulech C, Haspelova-Horvatovicovfi A (1986) Influence of the parasitic fungus Erysiphe graminis upon the assimilative pigments of barley plants during the individual stages of pathogenesis. Photobiochem Photobiophys 12:177–181
Pearce RB, Edwards PP, Green TL, Anderson PA, Fisher BJ, Carpenter TA (1997) Immobilized long-lived free radicals at the host–pathogen interface in sycamore (Acer pseudoplatanus L.). Physiol Mol Plant Pathol 50:371–390
Skipp RA, Selby C, Bailey JA (1977) Toxic effects of phaseollin on plant cells. Physiol Plant Pathol 10:221–227
Smith MT (1985) Quinones as mutagens, carcinogens, and anticancer agents: introduction and overview. J Toxicol Environ Health 16:665–672
Smith MM, Cruickshank IAM (1986) Dynamics of free pisatin elicitor accumulation in infection-droplets of Monilinia fructicola on pea endocarp. J Phytopath 117:301–311
Spoel SH, Johnson JS, Dong X (2007) Regulation of tradeoffs between plant defences against pathogens with different lifestyles. PNAS USA 104:18842–18847
Stobiecki M, Wojtaszek P, Gulewicz K (1997) Application of solid phase extraction for profiling of quinolisidine alkaloids and phenolic compounds in Lupinus albus. Phytochem Anal 8:153–158
Sweigard JA, Matthews DE, Van Etten HD (1986) Synthesis of the phytoalexin pisatin by a methyltransferase from pea. Plant Physiol 80:277–279
Testa B (1995) The metabolism of drugs and other xenobiotics. Academic, New York
Thomson RH (1987) Naturally occurring quinones III: recent advances. Chapman & Hall, London
Tivoli B, Banniza S (2007) Comparison of the epidemiology of Ascochyta blights on grain legumes. Eur J Plant Pathol 119:59–76
Van Etten HD, Bateman DF (1971) Studies on the mode of action of the phytoalexin phaseollin. Phytopathol 61:1363–1372
Van Gestelen P, Asard H, Horemans N, Caubergs RJ (1998) Superoxide-producing NAD(P)H oxidases in plasma membrane vesicles from elicitor responsive bean plants. Physiol Plant 104:653–660
Von Tiedemann A (1997) Evidence for a primary role of active oxygen species in induction of host cell death during infection of bean leaves with Botrytis cinerea. Physiol Mol Plant Pathol 50:151–166
Wasmann C, van Etten HD (1996) Transformation-mediated chromosome loss and disruption of a gene for pisatin demethylase decrease the virulence of Nectria haematococca on pea. Mol Plant–Microbe Interact 9:793–803
Weir GM (2005) Facultative parasites and host respiration III. Tissue which is actively parasitised. Mycopathologia 18(3):194–198
Zweier JL, Kuppusamy P (1988) Electron paramagnetic resonance measurements of free radicals in the intact beating heart: a technique for detection and characterization of free radicals in whole biological tissues. PNAS USA 85(15):5703–5707
Acknowledgements
This study was supported by the Polish Committee for Scientific Research (KBN, grant no. 3P06R05224).
Open Access
This article is distributed under the terms of the Creative Commons Attribution Noncommercial License which permits any noncommercial use, distribution, and reproduction in any medium, provided the original author(s) and source are credited.
Author information
Authors and Affiliations
Corresponding author
Additional information
Handling Editor: Peter Nick
Rights and permissions
Open Access This is an open access article distributed under the terms of the Creative Commons Attribution Noncommercial License (https://creativecommons.org/licenses/by-nc/2.0), which permits any noncommercial use, distribution, and reproduction in any medium, provided the original author(s) and source are credited.
About this article
Cite this article
Morkunas, I., Formela, M., Marczak, Ł. et al. The mobilization of defence mechanisms in the early stages of pea seed germination against Ascochyta pisi . Protoplasma 250, 63–75 (2013). https://doi.org/10.1007/s00709-012-0374-x
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00709-012-0374-x