Abstract
The changes in the formation of both the actin and the microtubular cytoskeleton during the differentiation of the embryo-suspensor in Sedum acre were studied in comparison with the development of the embryo-proper. The presence and distribution of the cytoskeletal elements were examined ultrastructurally and with the light microscope using immunolabelling and rhodamine-phalloidin staining. At the globular stage of embryo development extensive array of actin filaments is present in the cytoplasm of basal cell, the microfilament bundles generally run parallel to the long axis of basal cell and pass in close to the nucleus. Microtubules form irregular bundles in the cytoplasm of the basal cell. A strongly fluorescent densely packed microtubules are present in the cytoplasmic layer adjacent to the wall separating the basal cell from the first layer of the chalazal suspensor cells. At the heart-stage of embryo development, in the basal cell, extremely dense arrays of actin materials are located near the micropylar and chalazal end of the cell. At this stage of basal cell formation, numerous actin filaments congregate around the nucleus. In the fully differentiated basal cell and micropylar haustorium, the tubulin cytoskeleton forms a dense prominent network composed of numerous cross-linked filaments. In the distal region of the basal cell, a distinct microtubular cytoskeleton with numerous microtubules is observed in the cytoplasmic layer adjacent to the wall, separating the basal cell from the first layer of the chalazal suspensor cells. The role of cytoskeleton during the development of the suspensor in S. acre is discussed.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
The suspensor is a terminally differentiated embryonic region that anchors the embryo-proper (EP) to the surrounding maternal tissue and serves as a conduit for nutrients and growth regulators supporting embryo-proper development (for reviews, see Yeung and Meinke 1993; Schwartz et al. 1997). The suspensor degenerates by the end of embryogenesis, undergoes programmed cell death, and is not present in mature seeds (Raghavan 1986; Wredle et al. 2001). Previous investigations revealed that in many angiosperms, differentiation of the suspensor cells is frequently accompanied by polyploidization and polytenization of their nuclei (D'Amato 1984). In many plant species a high degree of ploidy during suspensor differentiation has been investigated: Phaseolus coccineus 8192 C (Brady 1973), Phaseolus hysterinus 4096n (Nagl 1976), Tropaeolum majus 2048C (Nagl 1976), Alisma plantago-aquatica 1024n (Bohdanowicz 1973), and Sedum acre 1024C (Kozieradzka-Kiszkurno and Bohdanowicz 2003). Thus polyploid and polytene cells have a particular significance for the function of the tissues and organs of which they are an integral part. The ultrastructural aspects of the suspensor development have been studied in many plants, e.g., Capsella bursa-pastoris (Schulz and Jensen 1969), P. coccineus (Yeung and Clutter 1979), A. plantago-aquatica, and Alisma lanceolatum (Bohdanowicz 1987), Paphiopedilium delenatii (Lee et al. 2006). In families such as Crassulaceae, Fumariaceae, Orchidaceae, Podostemaceae, Rubiaceae, Trapaceae, and Tropaeolaceae some suspensors develop haustoria that penetrate the endosperm and integuments (Mikesell 1990; Yeung and Meinke 1993; Raghavan 2006). So far, ultrastructural investigations on haustorial suspensors have been done only in T. majus (Nagl 1976), S. acre (Kozieradzka-Kiszkurno 2003). By contrast, there is a little information concerning the cytoskeleton of the suspensor in flowering plants available (Webb and Gunning 1991; Ye et al. 1997; Huang et al. 1998). In our preliminary investigation of Sedum, we observed that cytoskeleton is extremely abundant during the development of the suspensor. The embryo-suspensor in S. acre is a convenient model to study the cytoskeletal elements of highly polyploid plant cells in Crassulaceae. Therefore, the elucidation of presence of cytoskeletal elements in embryo-suspensor of S. acre can be helpful in understanding the role of cytoskeleton during the development of the suspensor in Crassulaceae. The purpose of this report is to investigate the development of embryo-suspensor of S. acre L. using the light and electron microscopy. The presence and distribution of the cytoskeletal elements were examined ultrastructurally and with the light microscope using immunolabelling and rhodamine-phalloidin staining.
Materials and methods
Plant material
Developing seeds of S. acre L. were collected from plants growing in natural habitats of Gdańsk in northern Poland. The study materials in various developmental stages were collected in summer months (June and July).
Transmission electron microscopy
The ovules in various developmental stages were fixed in 2.5% formaldehyde (prepared from paraformaldehyde) and 2.5% glutaraldehyde in 0.05 M cacodylate buffer (pH = 7.0) for 4 h at room temperature. The samples were rinsed in the same buffer and post-fixed in 1% osmium tetroxide in cacodylate buffer at 4°C overnight. Specimens were treated with 1% uranyl acetate in distilled water for 1 h, dehydrated in a graded acetone series, and embedded in Spurr's resin (Spurr 1969). Ultrathin (60–100 nm) sections were cut on with a diamond knife on a SORVALL MT 2B ultramicrotome. After contrasting with uranyl acetate and lead citrate, the sections were examined in a Philips CM 100 transmission electron microscope operating at 80 kV. Semithin (0.5–2 μm) sections for light microscopy were cut with glass knives and stained with 0.05% toluidine blue 0 in 1% sodium tetraborate.
Fluorescence assay of F-actin
The ovules were pretreated for 15–60 min in 400 μM MSB in PME buffer (50 mM PIPES, 1 mM MgCl2, 10 mM EGTA (pH = 6.8)). After pre-incubation, the ovules were washed in the PME buffer and fixed in 4% formaldehyde freshly prepared from paraformaldehyde in PME buffer with 5% DMSO for 4 h at room temperature. Next, ovules were washed in PME buffer and incubated in 0.33 μM rhodamine-phalloidin in PME buffer with 5% DMSO for 1.5 h. Following several rinses in PME buffer, nuclei were stained with DAPI. The embryo propers and suspensors were isolated from ovules under a stereomicroscope and placed immediately on a microscope slide.
Sample preparation for immunolocalization studies
For staining of microfilaments, ovules were fixed as described above and then used according to the procedure of Świerczyńska and Bohdanowicz (2003). To assay microtubules, ovules were fixed in 4% formaldehyde (freshly prepared from paraformaldehyde) and 0.25% glutaraldehyde in PME buffer for 4 h at room temperature. The fixed ovules were carried out using the procedure described by Bohdanowicz et al. (2005). After fixation, the plant material was rinsed three times in PME buffer, dehydrated in a graded ethanol series containing 10 mM DTT, infiltrated in Steedman's wax and sectioned into semithin sections. After that the sections were mounted on microscope slides coated with Mayer's egg albumen and dewaxed in absolute ethanol, dehydrated in ethanol—PBS series and washed in PBS.
Immunolabelling of tissue sections
Tissue sections were pre-incubated in PBS containing 0.1% BSA for 45 min at room temperature. For single staining of microtubules and microfilaments, preparations were incubated with primary antibodies, i.e., monoclonal antibody against mouse anti-β tubulin (Abcam; diluted 1:200) and monoclonal antibody against mouse actin (clone C4, ICN; diluted 1:1000) with PBS containing 0.1% BSA, each overnight at 4°C. After washing in PBS, the sections were incubated in secondary Alexa Fluor 488-conjugated goat anti-mouse antibody (Molecular Probes) diluted 1:800 in the same buffer for 4 h at room temperature. After labeling, the slides were rinsed in PBS and the nuclei stained by DAPI at solution of concentration 1 μg/ml for 10 min. Then the sections were treated with 0.01% toluidine blue in PBS for 10 min to diminish autofluorescence of the tissues. Finally sections were rinsed in PBS and mounted in anti-fade medium Citifluor AF1 (Agar). Control reactions were run without the primary antibody. Fluorescence observations were performed with a Nikon Eclipse E 800 epifluorescence microscope equipped with a Nikon cooled CCD camera.
Results
S. acre undergoes the Caryophyllad type of embryonic development. After the first division of the zygote, two cells are formed: a large basal cell (BC) and a smaller apical cell. The apical cell divides several times and develops into an EP and a chalazal suspensor, whereas the basal cell undergoes no division, becomes much enlarged, and forms a basal suspensor cell. The BC produces haustorial branches invading the micropyle and adjacent tissues, and protruding out of the ovule. Differentiation of the BC begins shortly after the division. The changes in the formation of both the actin and the microtubular cytoskeleton during the differentiation of the embryo-suspensor in S. acre were studied in comparison with the development of the embryo proper.
Globular-stage embryos
The fully differentiated suspensor is composed of a large, pear-shaped basal cell with the micropylar haustorium and a few chalazal cells (Fig. 1a). The nucleus is the most prominent organelle within the cell. The basal cell is filled with dense cytoplasm containing numerous ribosomes, mitochondria, plastids, profiles of endoplasmic reticulum, microbodies, dictyosomes, lipid droplets, and vesicles differing in size and content (Fig. 1b). At this stage of development the rhodamine-phalloidin staining indicates that fine actin filaments are present in the cytoplasm of the suspensor basal cell, numerous of which organize into long actin filaments and display clear parallel orientation from the micropylar to the chalazal pole of the basal cell (Fig. 1c). A large number of actin filaments occur at the chalazal pole of the basal cell near the wall, separating the basal cell from the chalazal suspensor cells (Fig. 1c). Furthermore, these actin filaments are more densely packed than those in the micropylar apex of the basal cell (Fig. 1c). Immunolabelling shows that a certain number of short microfilaments form a delicate network at the micropylar region of the basal cell (Fig. 1d). Some of these actin filaments run longitudinally or transversally to the long axis of the cell, but in the central and in the chalazal region of the basal cell we do not observe fluorescence of F-actin (Fig. 1d). However, we also note a large number of microfilaments which form an abundant and distinct array in the cytoplasm of the basal cell. Moreover, the microfilaments appear also to run pass the nucleus envelope and some bundles of actin filaments congregate around the nucleus (Fig. 1e). Simultaneously, numerous microfilaments aggregate and show strong fluorescence near the chalazal wall of the basal cell. (Fig. 1e). The fluorescence of the actin cytoskeleton of embryo-proper cells seems to be weak in comparison with the basal cell (Fig. 1d, e). Ultrastructural observations of microfilaments distribution confirm the results obtained by immunofluorescent staining of actin at the level of light microscopy. Bundles of microfilaments are sometimes visible near other cell organelles such as: endoplasmic reticulum, mitochondria, dictyosomes (Fig. 1f). At this time the bundles of tubulin form a prominent network in the cytoplasm of the basal cell (Fig. 2a). A strongly fluorescent densely packed microtubules are present in the cytoplasmic layer adjacent to the wall separating the basal cell from the first layer of the chalazal suspensor cells (Fig. 2a). At this stage of development, this wall is perforated by compound plasmodesmata associated with unusual electron-dense dome on the basal cell side (Fig. 2b). Microtubules gather near the wall separating the basal cell from the first layer of the chalazal suspensor cells (Fig. 2c). As development progresses, the microtubules are more abundant and still form a prominent network which extends from micropylar to chalazal apex of the basal cell (Fig. 2d). The suspensor basal cell continues to enlarge. A single large nucleus locates centrally. Strong concentration of the microtubules is visible nearby the nucleus (Fig. 2d). Again, at the chalazal end of suspensor basal cell, a high concentration of the microtubules is present and shows an intensive fluorescence (Fig. 2d). Simultaneously, the micropylar haustorium of the basal cell is already strongly developed and ramifies in the integumentary tissues (Fig. 2d). A large amount of the microtubules is observed in the micropylar haustorium cytoplasm (Fig. 2e). The microtubules form a fine meshwork of filaments near the micropylar tip of the suspensor basal cell. (Fig. 2f).
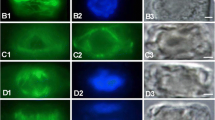
a–f The globular stage of S. acre embryo-suspensor development. (a, c–e) Light micrographs, (b, f) Electron micrographs. a Semithin section showing the large basal cell (BC) with the micropylar haustorium (MH), two layers of the chalazal suspensor (CHS) and the embryo-proper (EP). b The basal cell has a dense cytoplasm and a prominent nucleus (N). Note a numerous of ribosomes, mitochondria, plastids, profiles of the endoplasmic reticulum, dictyosomes, lipid droplets, and vesicles differing in size and content in the cytoplasm of the BC. c Image of F-actin rhodamine-phalloidin assay. F-actin filaments (arrow) run parallel to the long axis of the basal cell. A large number of actin filaments occur at the chalazal pole of the basal cell near the wall, separating the basal cell from the chalazal suspensor cells (arrowhead). (d, e) Images of F-actin by antibody immunofluorescence labeling (yellow); nuclei by DAPI staining (blue). d Actin network (arrow) at the micropylar apex of the basal cell. e Abundant bundles of actin filaments. The microfilaments concentrate deep in the cytoplasm. Some of them assemble close to the nucleus (N) and the chalazal pole occurs attached to the CHS (arrows). f Electron micrograph showing the bundles of microfilaments (MF), mitochondria (M), dictyosomes (D), endoplasmic reticulum
The globular stage of S. acre embryo-suspensor development; a, d–f images of tubulin by antibody immunolabelling (yellow), nuclei by DAPI staining (blue); b, c electron micrographs. a A longitudinal section showing the large basal cell with the micropylar haustorium, two layers of the chalazal suspensor cells and the embryo-proper. The bundles of tubulin form a prominent network in the cytoplasm of the basal cell. Numerous microtubules distribute at the chalazal end of the basal cell and form a dense, strongly fluorescence arrays of tubulin (arrow). b Fragment of the wall separating the basal cell (BC) from the first layer of the chalazal suspensor (CHS). Note the numerous unusual, compound plasmodesmata (PD). c Unusual plasmodesma (PD) at a higher magnification. Note an electron-dense dome associated with compound plasmodesma. The microtubules (arrows) are located near the cell wall (W) in the cytoplasm of the basal cell (BC); CHS. d In the basal cell, a prominent tubulin network spread from micropylar to chalazal end of the cell. Numerous bundles of microtubules locate at the chalazal end of the basal cell. At this stage, some bundles of microtubules congregate near the nucleus (arrow). Note a large amount of microtubules in the micropylar haustorium cytoplasm (arrowheads). e Fragment of the micropylar haustorium of the basal cell. Majority of microtubules distribute along the haustorium. f The micropylar end of the basal cell (BC). The tubulin cytoskeleton forms an intricate network (arrow)
Heart-stage embryos
When the embryo proper reaches the heart stage of development, the BC undergoes the full development. The fully developed suspensor is built of a large pear-shaped basal cell and two to four chalazal cells in two layers. At this phase of development, the micropylar anucleate haustorium of the BC is already strongly developed and ramifies in the integumentary tissues (Fig. 3a). At this stage, the organization of rhodamine-phalloidin staining microfilaments is similar to that of the BC of the globular embryo with more abundant microfilaments localized in the micropylar apex of the basal cell (Fig. 3b). These microfilaments form a dense actin array (Fig. 3b). Significantly, a number of parallel-oriented actin filaments increase also at the central part of the basal cell. A little change can be seen in the distribution of microfilaments at the chalazal apex of the basal cell (Fig. 3b). Immunofluorescent F-actin visualization reveals significant changes in F-actin organization at the micropylar part of the basal cell. At this time a delicate F-actin network reorganizes into longitudinally oriented actin bundles. These actin bundles become the dominant feature at the micropylar pole of the basal cell (Fig. 3c). However, some actin filaments are also visible in the central part of the basal cell (Fig. 3d). Once again, the microfilaments concentrate close to the nucleus. F-actin filaments appear to aggregate and organize into thick actin cables which arrange circular in the cytoplasm of the basal cell (Fig. 3d). As in the previous stage of embryo development, the ultrastructural observations on distribution of both the microfilaments and microtubules confirm the results obtained by immunofluorescent staining of actin and tubulin at the level of light microscopy. At higher magnification, microfilaments (Fig. 3e) and microtubules (Fig. 3e, g) are found in the cytoplasm near the nucleus. At this time immunoassay shows a prominent network of microtubules in the cytoplasm of the basal cell (Fig. 3f). At this late stage (Fig. 4a), a new distribution pattern of actin cytoskeleton can be found within the cytoplasm of the BC. Microfilaments remain still very abundant and form an intricate actin network which extends from the micropylar to the chalazal pole of the BC (Fig. 4b). However, microfilaments occupying the cytoplasm of the basal cell organize into randomly aligned bundles of actin filaments (Fig. 4b). Simultaneously, the number of parallel-oriented fine actin filaments decrease at the micropylar pole of the BC (Fig. 4c). Moreover, F-actin surrounding the nucleus disappears, only a few dense actin arrays can be seen close to the nucleus surface (Fig. 4c). In addition, only at this stage of development, we note brightly fluorescing compact arrays of actin filaments concentrating near the edges of the BC. Some bundles of actin filaments seem to radiate from these actin arrays (Fig. 4c). At this time a rich actin cytoskeleton is present in the cytoplasm of the micropylar haustorium (Fig. 4d). Majority of these actin filaments are oriented longitudinally to the long axis of the haustorial branch, although a subtle net of microfilaments is also visible (Fig. 4d).
The early heart stage of S. acre embryo-suspensor development. a–d, f Light micrographs; e, g electron micrographs. a A longitudinal section stained with toluidine blue 0. b A light micrograph showing the fluorescence pattern after rhodamine-phalloidin staining. In the basal cell, a dense array of actin filaments locates near the micropylar (arrowhead) and chalazal (arrow) end of the cell. Bundles of F-actin filaments arrange longitudinally. c–d, f Immunostaining of microfilaments in the basal cell. c The majority of actin filaments are distributed at the micropylar pole of the basal cell and form a delicate actin network (arrow). d Thick actin cables of F-actin arrange circular in the cytoplasm of the basal cell (arrows). Some microfilaments congregate the nucleus (arrowheads). e The bundles of microfilaments (MF) and microtubules (arrows) are localized near the nucleus (N). f A prominent network of microtubules in the cytoplasm of the basal cell. g The microtubules (MT) are congregated near the nucleus; N nucleus
Light micrographs of the late stage of S. acre embryo-suspensor development (a–d). a A longitudinal section through the embryo-suspensor and the embryo-proper. b, c, d Immunoassay of microfilaments in the basal cell. b Immunofluorescence image showing a netlike distribution of actin from the micropyle to the chalazal pole. c Intricate microfilaments network is readily observed at the micropylar end of the suspensor basal cell (arrows). At this stage only, a brightly fluorescing actin arrays can be seen near the edges of the basal cell (arrowhead); N nucleus d Fragment of micropylar haustorium of the basal cell. Section showing the fluorescence pattern of actin cytoskeleton. Majority of F-actin filaments locate along the haustorium (arrows)
Discussion
In the majority of angiosperm taxa, the embryo-suspensor is a fast-growing and short-living organ with important functions during plant embryogenesis (for review, see Yeung and Meinke 1993). Ultrastructural and experimental investigations clearly indicate that it can serve as a channel for nutrient flow and may provide unique metabolites for the growth of the embryo-proper (for review, see Yeung and Meinke 1993; Schwartz et al. 1997; Lee et al. 2006). In some plants, prominent haustoria arise as lateral projections of suspensor cells and invade the endosperm and surrounding maternal tissues (Raghavan 2006). The formation of a huge haustorial suspensor also accompanies embryogenesis in S. acre and some other genera of Crassulaceae (Mauritzon 1933). An interesting feature of many plant species is that suspensor basal cell differentiation is accompanied by polyploidization and polytenization. Polyploid cells are characteristic for secretory and/or nutritional organs limited to ovary tissues (synergids, antipodals, endosperm, embryo-suspensor). The nuclear DNA content of polyploid cells is sometimes a hundred or a thousand times higher than the DNA content of diploid cells of the same tissues (Brodsky and Uryvayeva 1985). It has been established that the plant cytoskeleton plays important roles in plant growth and development. The organization of the cytoskeleton has been reported during megasporogenesis and megagametogenesis (Willemse and van Lammeren 1988; Bednara et al. 1990; Webb and Gunning 1991; Zee and Ye 1995; Ye et al. 1997; Huang et al. 1998; Tung et al. 1999, 2000; Świerczyńska and Bohdanowicz 2003; Płachno et al. 2010). Thus far, information about ultrastructure and cytoskeleton in highly polyploid haustorial suspensors is scarce. In the present study, we show the visualization of F-actin and tubulin within the embryo-suspensor of S. acre. During the development of suspensor of S. acre, the actin filaments tend to orient in the same direction as the long axis of the basal cell. Similar microfilament arrays have been reported in the embryo-suspensor of the Nun orchid Phaius tankervilliae (Ye et al. 1997). The functional significance of the high concentration of microfilaments near the micropylar apex of the basal cell in P. tankervilliae is not known. However, Ye et al. (1997) suggest this pattern of distribution is similar to that of the pollen at the onset of pollen germination and connected with creation of predetermined site for the elongation of the suspensor later on. In our opinion, this network of actin filaments that occurs at the micropylar tip of the basal cell in S. acre may be associated primarily with the cytoplasmic streaming movement of the cytoplasm between this cell and its haustorium. However, the role of actin filaments in the formation and expansion of the haustorium cannot be excluded, especially in the early stage of development of basal cell. On the other hand, we note that the actin network and numerous mitochondria observed at the micropylar end of the basal cell are supposedly connected with wall ingrowths, formed and functioning there. Similar observations of microfilaments have been made in the endosperm chalazal haustorium of Rhinanthus serotinus (Świerczyńska and Bohdanowicz 2003) and in the syncytia of Utricularia (Płachno et al. 2010). The occurrence of such ingrowths, greatly increasing the absorptive surface of the plasma membrane, seems to be a common feature of these cells and has been reported previously for several other species (Gunning and Pate 1969; Yeung and Meinke 1993). In S. acre the presence of the wall ingrowths, and the abundance of mitochondria, endoplasmic reticulum, and plastids clearly indicate that the suspensor of S. acre indeed has structural specialization similar to those of other flowering plants. In S. acre, cytochemical results on the composition and distribution of macromolecules (proteins, insoluble polysaccharides, nucleic acids, and lipids) at various stages of the development of the embryo-proper and suspensor (Kozieradzka-Kiszkurno and Bohdanowicz 2006), and analysis of the suspensor ultrastructure in S. acre (Kozieradzka-Kiszkurno 2003) show that the basal cell is a site of intense metabolic activity. We observed a large amount of actin filaments and numerous profiles of endoplasmic reticulum, dictyosomes, plastids occurring at the chalazal pole of the basal cell near the wall, separating the basal cell from the chalazal suspensor cells. Our ultrastructural study indicates the presence of microfilaments and microtubules near the chalazal pole of the basal cell. It is presumably connected with the fact, that in the wall, separating the cell from the first layer of the chalazal suspensor cells there are unusual, compound plasmodesmata. An electron-dense material, which appears from the basal cell side, might be a kind of a filter, which controls the transfer of the nutrients (Kozieradzka-Kiszkurno and Bohdanowicz 2010). Moreover, in S. acre, the frequency of numerous polysomes suggests active protein synthesis which would be necessary for rapid cell growth. Microfilaments are the major cytoskeletal component of plant cells (Parthasarathy et al. 1985), and their importance in plant growth and development is well recognized (Staiger and Schliwa 1987; Hussey et al. 2006). The best understood function of microfilaments in plants is the generation of motive force for cytoplasmic streaming (Williamson 1986). In the few reports about microfilament organization (Derksen et al. 1986; Staiger and Schliwa 1987; Staiger et al. 2000), a distinct actin network connected to a meshwork of microfilaments enveloping the cell nucleus has been described. In the S. acre, enlargement of the basal cell nucleus is one of the first indications of its specialization. Multiplication of nuclear DNA content is one of the most common processes connected with cell differentiation in plants. The perinuclear distribution actin organization in the suspensor basal cell indicates that, besides other functions, microfilaments may play a role in nuclear positioning. The participation of the cytoskeleton and in particular actin filaments in mRNA transport has been reported (Nasmyth and Jansen 1997; Muench et al. 1998; Muench and Park 2006). The role of actin in the movement and localization of organelles has been confirmed by numerous fluorescence observations (Boevink et al. 1998; Kandasamy and Meagher 1999). Besides microfilaments, the other major components of the cytoskeleton, microtubules, also play an important role. In S. acre, the microtubules form irregular bundles in suspensor basal cell cytoplasm, while in Cymbidium sinense the microtubule of the suspensor cells tend to orient in the same direction as the long axis of the cell during initial expansion. When the suspensor cells begin to elongate, their cortical microtubules reorient from a longitudinal to a transverse direction (Huang et al. 1998). Changes in the microtubule orientation have been correlated with changes in the cell shape (Cyr 1994) such as in the suspensor cell of Phaius tankervilleae (Ye et al. 1997).
These studies clearly indicate that cytoskeletal elements play an important role during cell expansion and elongation. In S. acre the microtubules congregate near the nucleus. Microtubules play an important role in the regulation of cell growth (Derksen et al. 1990). The cytoskeleton performs an important function in many cellular processes, like cellular signaling, organelle motility, and also subcellular compartmentation during plant growth and development (Nasmyth and Jansen 1997; Grolig 1998; Nick 1999; Ramachandran et al. 2000; Baluška et al. 2001; Kost et al. 2002; Mathur and Hülskamp 2002; Wasteneys and Yang 2004). Therefore, analyses of suspensors in S. acre are potentially interesting for plant development and evolution.
Abbreviations
- BC:
-
Basal cell
- BSA:
-
Bovine serum albumin
- CHS:
-
Chalazal suspensor cells
- DAPI:
-
4′ 6′-Diamidino-2-phenylindole dihydrochloride
- DMSO:
-
Dimethyl sulfoxide
- DTT:
-
Dithiothreitol
- EGTA:
-
Ethylene glycol-bis (2-aminoethylether)-N,N,N′,N′-tetraacetic acid
- EP:
-
Embryo-proper
- MH:
-
Micropylar haustorium
- MSB:
-
m-Maleimidobenzoic acid N-hydroxysuccinimide ester
- PBS:
-
Phosphate-buffered saline
- PIPES:
-
Piperazine-N,N′-bis (2-ethanesulfonic acid)
References
Baluška F, Jasik J, Edelmann HG, Salajova T, Volkmann D (2001) Latrunculin B-induced plant dwarfism: plant cell elongation is F-actin-dependent. Dev Biol 231:113–124
Bednara J, Willemse MTM, van Lammeren AAM (1990) Organization of the actin cytoskeleton during megasporogenesis in Gasteria verrucosa visualized with fluorescent-labelled phalloidin. Acta Bot Neerl 39:43–48
Boevink P, Oparka K, Santa Cruz S, Martin B, Betteridge A, Hawes C (1998) Stacks on tracks: the plant Golgi apparatus traffics on an actin/ER network. Plant J 15:441–447
Bohdanowicz J (1973) Karyological anatomy of the suspensor in Alisma L. I. Alisma plantago-aquatica L. Acta Biol Crac Ser Bot 16:235–248
Bohdanowicz J (1987) Alisma embryogenesis: the development and ultrastructure of the suspensor. Protoplasma 137:71–83
Bohdanowicz J, Szczuka E, Świerczyńska J, Sobieska J, Kościńska-Pająk M (2005) The distribution of microtubules during regular and disturbed microsporogenesis and pollen grain development in Gagea lutea (L.) Ker.-Gaw. Acta Biol Cracov Ser Bot 47:89–96
Brady T (1973) Feulgen cytophotometric determination of the DNA content of the embryo proper and suspensor cells of Phaseolus coccineus. Cell Differ 2:65–75
Brodsky VYA, Uryvayeva JV (1985) Genome multiplication in growth and development. Biology of polyploid and polytene cells. Cambridge University Press, Cambridge
Cyr RJ (1994) Microtubules in plant morphogenesis: role of the cortical array. Ann Rev Cell Biol 10:153–180
D'Amato F (1984) Embryology of angiosperms. In: Johri BM (ed) Role of polyploidy in reproductive organs and tissues. Springer, Berlin, pp 519–566
Derksen J, Trass JA, Oostendorp T (1986) Distribution of actin filaments in differentiating cells of Equisetum hyemale root tips. Plant Sci 43:77–81
Derksen J, Wilm EHA, Pierson ES (1990) The plant cytoskeleton: in significance in plant development. Acta Bot Neerl 39:1–18
Grolig F (1998) Nuclear centering in Spirogyra: force integration by microfilaments along microtubules. Planta 204:54–63
Gunning BES, Pate JS (1969) “Transfer cells”: plant cells with wall ingrowths, specialized in relation to short distance transport of solutes—their occurrence, structure, and development. Protoplasma 68:107–133
Huang BQ, Ye XL, Yeung E, Zee SY (1998) Embryology of Cymbidium sinense: the microtubule organization of early embryos. Ann Bot 81:741–750
Hussey PJ, Ketelaar T, Deeks MJ (2006) Control of the actin cytoskeleton in plant cell growth. Annu Rev Plant Biol 57:109–125
Kandasamy M, Meagher RB (1999) Actin-organelle interaction: association with chloroplasts in Arabidopsis leaf mesophyll cells. Cell Motil Cytoskeleton 44:110–118
Kost B, Bao YO, Chua NH (2002) Cytoskeleton and plant organogenesis. Philos Trans R Soc Lond 357:777–789
Kozieradzka-Kiszkurno M (2003) Development, ultrastructure and cytochemistry of embryo-suspensor in Sedum acre L. (Crassulaceae). PhD Thesis, University of Gdańsk, Gdańsk, Poland
Kozieradzka-Kiszkurno M, Bohdanowicz J (2003) Sedum acre embryogenesis: polyploidization in the suspensor. Acta Biol Cracov Ser Bot 45:159–163
Kozieradzka-Kiszkurno M, Bohdanowicz J (2006) Development and cytochemistry of the embryo suspensor in Sedum. Acta Biol Cracov Ser Bot 48:67–72
Kozieradzka-Kiszkurno M, Bohdanowicz J (2010) Unusual electron-dense dome associates with compound plasmodesmata in the embryo-suspensor of genus Sedum (Crassulaceae). Protoplasma 247:117–120. doi:10.1007/s00709-010-0133-9
Lee YI, Yeung EC, Lee N, Chung MC (2006) Embryo development in the Lady's Slipper Orchid, Paphiopedilum delenatii, with emphasis on the ultrastructure of the suspensor. Ann Bot 98:1311–1319
Mathur J, Hülskamp M (2002) Microtubules and microfilaments in cell morphogenesis in higher plants. Curr Biol 12:669–676
Mauritzon J (1933) Studien über die Embryologie der Familien Crassulaceae und Saxifragaceae. Thesis, University of Lund, Lund
Mikesell J (1990) Anatomy of terminal haustoria in the ovule of plantain (Plantago major L.) with taxonomic comparison to other angiosperm taxa. Bot Gaz 151:452–464
Muench DG, Park NI (2006) Messages on the move: the role of the cytoskeleton in mRNA localization and translation in plant cells. Can J Bot 84:572–580
Muench DG, WU Y, Coughlan SJ, Okita TW (1998) Evidence for a cytoskeleton-associated binding site involved in prolamine mRNA localization to the protein bodies in rice endosperm tissue. Plant Physiol 116:559–569
Nagl W (1976) Early embryogenesis in Tropaeolum majus L.: ultrastructure of the embryo-suspensor. Biochem Physiol Pflanz 170:253–260
Nasmyth K, Jansen RP (1997) The cytoskeleton in mRNA localization and cell differentiation. Curr Opin Cell Biol 9:396–400
Nick P (1999) Signals, motors, morphogenesis—the cytoskeleton in plant development. Plant Biol 1:169–179
Parthasarathy MV, Perdue JD, Witted AJ, Alvernaz J (1985) Actin network as a normal component of the cytoskeleton in many vascular plant cells. Am J Bot 77:1318–1323
Płachno BJ, Świątek P, Kozieradzka-Kiszkurno M (2010) The F-actin cytoskeleton in syncytia from non-clonal progenitor cells. Protoplasma. doi:10.1007/s00709-010-0209-6
Raghavan V (1986) Embryogenesis in angiosperms. A developmental and experimental study. Cambridge University Press, Cambridge
Raghavan V (2006) Double fertilization—embryo and endosperm development in flowering plants. Springer, Berlin
Ramachandran S, Christensen HEM, Ishimaru Y, Dong CH, Chao-Ming W, Cleary AL, Chua NH (2000) Profilin plays a role in cell elongation, cell shape maintenance, and flowering in Arabidopsis. Plant Physiol 124:1637–1647
Schulz SR, Jensen WA (1969) Capsella embryogenesis: the suspensor and the basal cell. Protoplasma 67:139–163
Schwartz BW, Vernon DA, Meinke DW (1997) Development of the suspensor: differentiation, communication and programmed cell death during plant embryogenesis. In: Vasil B (ed) Cellular and molecular biology of plant seed development. Kluwer, Dordrecht, pp 53–72
Spurr AR (1969) A low-viscosity epoxy resin embedding medium for electron microscopy. J Ultrastruct Res 26:31–43
Staiger CJ, Schliwa M (1987) Actin localization and function in higher plants. Protoplasma 148:80–86
Staiger CJ, Baluška F, Volkmann D, Barlow P (eds) (2000) Actin: a dynamic framework for multiple plant cell functions. Kluwer, Dordrecht
Świerczyńska J, Bohdanowicz J (2003) Microfilament cytoskeleton of endosperm chalazal haustorium of Rhinanthus serotinus (Scrophulariaceae). Acta Biol Crac Ser Bot 45:143–148
Tung SH, Ye XL, Yeung EC, Zee SY (1999) Ultrastructural aspects of megasporogenesis in Cymbidium sinense (Orchidaceae). Lindleyana 14:178–192
Tung SH, Ye XL, Zee SY, Yeung EC (2000) The microtubular cytoskeleton during megasporogenesis in the Nun orchid, Phaius tankervilliae. New Phytol 146:503–513
Wasteneys GO, Yang Z (2004) New views on the plant cytoskeleton. Plant Physiol 136:3884–3891
Webb MC, Gunning BES (1991) The microtubular cytoskeleton during development of the zygote, proembryo and free-nuclear endosperm in Arabidopsis thaliana (L.) Heynh. Planta 184:187–195
Willemse MTM, van Lammeren AAM (1988) Structure and function of the microtubular cytoskeleton during megasporogenesis and embryo sac development in Gasteria verrucosa (Mill.) H. Duval. Sex Plant Reprod 82:631–634
Williamson RE (1986) Organelle movements along actin filaments and microtubules. Plant Physiol 82:631–634
Wredle U, Walles B, Hakman I (2001) DNA fragmentation and nuclear degradation during programmed cell death in the suspensor and endosperm of Vicia faba. Int J Plant Sci 162:1053–1063
Ye XL, Zee SY, Yeung EC (1997) Suspensor development in the Nun orchid, Phaius tankervilliae. Int J Plant Sci 158:704–712
Yeung EC, Clutter ME (1979) Embryogeny of Phaseolus coccineus: the ultrastructure and development of the suspensor. Can J Bot 57:120–136
Yeung EC, Meinke DW (1993) Embryogenesis in angiosperms: development of the suspensor. Plant Cell 5:1371–1381
Zee SY, Ye XL (1995) Changes in the pattern of organization of the microtubular cytoskeleton during megasporogenesis in Cymbidium sienense. Protoplasma 185:170–177
Conflicts of interest statement
The authors declare that they have no conflict of interest.
Open Access
This article is distributed under the terms of the Creative Commons Attribution Noncommercial License which permits any noncommercial use, distribution, and reproduction in any medium, provided the original author(s) and source are credited.
Author information
Authors and Affiliations
Corresponding author
Additional information
Handling Editor: Marisa Otegui
Rights and permissions
Open Access This is an open access article distributed under the terms of the Creative Commons Attribution Noncommercial License (https://creativecommons.org/licenses/by-nc/2.0), which permits any noncommercial use, distribution, and reproduction in any medium, provided the original author(s) and source are credited.
About this article
Cite this article
Kozieradzka-Kiszkurno, M., Świerczyńska, J. & Bohdanowicz, J. Embryogenesis in Sedum acre L.: structural and immunocytochemical aspects of suspensor development. Protoplasma 248, 775–784 (2011). https://doi.org/10.1007/s00709-010-0248-z
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00709-010-0248-z