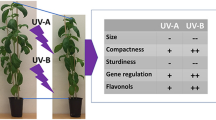
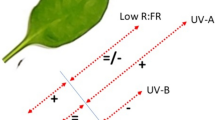
Abstract
Long-term effects of ultraviolet (UV) radiation on flavonoid biosynthesis were investigated in Arabidopsis thaliana using the sun simulators of the Helmholtz Zentrum München. The plants, which are widely used as a model system, were grown (1) at high photosynthetically active radiation (PAR; 1,310 µmol m−2 s−1) and high biologically effective UV irradiation (UV-BBE 180 mW m−2) during a whole vegetative growth period. Under this irradiation regime, the levels of quercetin products were distinctively elevated with increasing UV-B irradiance. (2) Cultivation at high PAR (1,270 µmol m−2 s−1) and low UV-B (UV-BBE 25 mW m−2) resulted in somewhat lower levels of quercetin products compared to the high-UV-BBE conditions, and only a slight increase with increasing UV-B irradiance was observed. On the other hand, when the plants were grown (3) at low PAR (540 µmol m−2 s−1) and high UV-B (UV-BBE 180 mW m−2), the accumulation of quercetin products strongly increased from very low levels with increasing amounts of UV-B but the accumulation of kaempferol derivatives and sinapoyl glucose was less pronounced. We conclude (4) that the accumulation of quercetin products triggered by PAR leads to a basic UV protection that is further increased by UV-B radiation. Based on our data, (5) a combined effect of PAR and different spectral sections of UV radiation is satisfactorily described by a biological weighting function, which again emphasizes the additional role of UV-A (315–400 nm) in UV action on A. thaliana.





Similar content being viewed by others
References
Berkelaar EJ, Omrod DP, Hale BA (1994) The influence of photosynthetically active radiation on the effects of ultraviolet-B radiation on Arabidopsis thaliana. Photochem Photobiol 64:110–116
Bieza K, Lois R (2001) An Arabidopsis mutant tolerant to lethal ultraviolet-B levels shows constitutively elevated accumulation of flavonoids and other phenolics. Plant Physiol 126:1105–1115
Bornman JF, Teramura AH (1993) Effects of ultraviolet-B radiation on terrestrial plants. In: Young AR, Björn LO, Moan J, Nultsch W (eds) Environmental UV photobiology. Plenum, New York, pp 427–471
Burchard P, Bilger W, Weissenböck G (2000) Contribution of hydroxycinnamates and flavonoids to epidermal shielding of UV-A and UV-B radiation in developing rye primary leaves as assessed by ultraviolet-induced chlorophyll fluorescence measurements. Plant Cell Environ 23:1373–1380
Caldwell MM (1971) Solar UV radiation and the growth and development of higher plants. In: Giese AC (ed) Photophysiology, vol 6. Academic, New York, pp 131–177
Caldwell MM, Flint SD (1994) Lighting considerations in controlled environments for nonphotosynthetic plant responses to blue and ultraviolet radiation. In: Tibbitts TW (ed) International Lighting in Controlled Environments Workshop. NASA-CP-95-3309, pp. 113–124
Caldwell MM, Bornman JF, Ballaré CL, Flint SD, Kulandaivelu G (2007) Terrestrial ecosystems, increased solar ultraviolet radiation, and interactions with other climate change factors. Photochem Photobiol Sci 6:252–266
Casati P, Walbot V (2003) Gene expression profiling in response to ultraviolet radiation in maize genotypes with varying flavonoid content. Plant Physiol 132:1739–1754
Cooley NM, Truscott HMF, Holmes MG, Attridge TH (2000) Outdoor ultraviolet polychromatic action spectra for growth responses of Bellis perennis and Cynosurus cristatus. J Photochem Photobiol B 59:64–71
Dunaeva M, Adamska I (2001) Identification of genes expressed in response to light stress in leaves of Arabidopsis thaliana using RNA differential display. Eur J Biochem 268:5521–5529
Flint SD, Caldwell MM (2003) Field testing of UV biological weighting functions for higher plants. Physiol Plant 117:145–153
Ghetti F, Herrmann H, Häder D-P, Seidlitz HK (1999) Spectral dependence of the inhibition of photosynthesis under simulated global radiation in the unicellular green alga Dunaliella salina. J Photochem Photobiol B 48:166–173
Giordano CV, Mori T, Sala OE, Scopel AL, Caldwell MM, Ballaré CL (2003) Functional acclimation to solar UV-B radiation in Gunnera magellanica, a native plant species of southernmost Patagonia. Plant Cell Environ 26:2027–2036
Glombitza S, Dubuis P-H, Thulke O, Welzl G, Bovet L, Götz M, Affenzeller M, Geist B, Hehn A, Asnaghi C, Ernst D, Seidlitz HK, Gundlach H, Mayer KF, Martinoia E, Werck-Reichhart D, Mauch F, Schäffner AR (2004) Crosstalk and differential response to abiotic and biotic stressors reflected at the transcriptional level of effector genes from secondary metabolism. Plant Mol Biol 54:817–835
Ibdah M, Krins A, Seidlitz HK, Heller W, Strack D, Vogt T (2002) Spectral dependence of flavonol and betacyanin accumulation in Mesembryanthemum crystallinum under enhanced UV radiation. Plant Cell Environ 25:1145–1154
Izaguirre MM, Scopel AL, Baldwin IT, Ballaré CL (2003) Convergent responses to stress. Solar ultraviolet-B radiation and Manduca sexta herbivory elicit overlapping transcriptional responses in field-grown plants of Nicotiana longiflora. Plant Physiol 132:1755–1767
Jansen MAK, Gaba V, Greenberg BM (1998) Higher plants and UV-B radiation: balancing damage, repair and acclimation. Trends Plant Sci 3:131–135
Jordan BR (1996) The effects of ultraviolet-B radiation on plants: a molecular perspective. Adv Bot Res 22:97–162
Kaffarnik F, Seidlitz HK, Obermaier J, Sandermann H, Heller W (2006) Environmental and developmental effects on the biosynthesis of UV-B screening pigments in Scots pine (Pinus sylvestris L.) needles. Plant Cell Environ 29:1484–1491
Krizek DT (2004) Influence of PAR and UV-A in determining plant sensitivity and photomorphogenic responses to UV-B radiation. Photochem Photobiol 79:307–315
Langebartels C, Schraudner M, Heller W, Ernst D, Sandermann H (2002) Oxidative stress and defense reactions in plants exposed to air pollutants and UV-B radiation. In: Inzé D, Van Montagu M (eds) Oxidative stress in plants. Taylor & Francis, London, pp 105–135
Mazza CA, Battista D, Zima AM, Szwarcberg-Bracchitta M, Giordano CV, Acevedo A, Scopel AL, Ballaré CL (1999) The effects of solar ultraviolet-B radiation on the growth and yield of barley accompanied by increased DNA damage and antioxidant responses. Plant Cell Environ 22:61–70
McKenzie RL, Aucamp PJ, Bais A, Björn LO, Ilyas M (2007) Changes in biologically-active ultraviolet radiation reaching the Earth’s surface. Photochem Photobiol Sci 6:218–231
Meijkamp BB, Doodeman G, Rozema J (2001) The response of Vicia faba to enhanced UV-B radiation under low and near ambient PAR levels. Plant Ecol 154:137–146
Quaite FE, Sutherland BM, Sutherland JC (1992) Action spectrum for DNA damage in alfalfa lowers predicted impact of ozone depletion. Nature 358:576–578
Ries G, Heller W, Puchta H, Sandermann H, Seidlitz HK, Hohn B (2000) Elevated UV-B radiation reduces genome stability in plants. Nature 406:98–101
Rossel JB, Wilson IW, Pogson BJ (2002) Global changes in gene expression in response to high light in Arabidopsis. Plant Physiol 130:1109–1120
Rousseaux MC, Ballaré CL, Giordano CV, Scopel AL, Zima AM, Szwarcberg-Bracchitta M, Searles PS, Caldwell MM, Diaz SB (1999) Ozone depletion and UVB radiation: Impact on plant DNA damage in southern South America. Proc Natl Acad Sci USA 96:15310–15315
Ryan KG, Markham KR, Bloor SJ, Bradley JM, Mitchell KA, Jordan BR (1998) UVB radiation induced increase in quercetin : kaempferol ratio in wild-type and transgenic lines of Petunia. Photochem Photobiol 68:323–330
Ryan KG, Swinny EE, Winefield C, Markham KR (2001) Flavonoids and UV photoprotection in Arabidopsis mutants. Z Naturforsch 56c:745–754
Ryan KG, Swinny EE, Markham KR, Winefield C (2002) Flavonoid gene expression and UV photoprotection in transgenic and mutant Petunia leaves. Phytochemistry 59:23–32
Sancar A (1994) Structure and function of DNA photolyase. Biochemistry 33:2–9
Schnitzler J-P, Jungblut TP, Heller W, Köfferlein M, Hutzler P, Heinzmann U, Schmelzer E, Ernst D, Langebartels C, Sandermann H (1996) Tissue localization of u.v.-B-screening pigments and of chalcone synthase mRNA in needles of Scots pine seedlings. New Phytol 132:247–258
Schnitzler J-P, Langebartels C, Heller W, Liu J, Lippert M, Döhring T, Bahnweg G, Sandermann H (1999) Ameliorating effect of UV-B radiation on the response of Norway spruce and Scots pine to ambient ozone concentrations. Global Change Biol 5:83–94
Stapleton AE (1992) Ultraviolet radiation and plants: burning questions. Plant Cell 4:1353–1358
Sullivan JH, Gitz DC III, Liu-Gitz L, Xu C, Gao W, Slusser J (2007) Coupling short-term changes in ambient UV-B levels with induction of UV-screening compounds. Photochem Photobiol 83:863–870
Thiel S, Döhring T, Köfferlein M, Kosak A, Martin P, Seidlitz HK (1996) A phytotron for plant stress research: how far can artificial lighting compare to natural sunlight? J Plant Physiol 148:456–463
Turunen M, Heller W, Stich S, Sandermann H, Sutinen M-L, Norokorpi Y (1999) The effects of UV exclusion on the soluble phenolics of young Scots pine seedlings in the subarctic. Environ Pollut 106:219–228
Weatherhead EC, Andersen SB (2006) The search for signs of recovery of the ozone layer. Nature 441:39–45
Yao Y, Yang Y, Ren J, Li C (2006) UV-spectra dependence of seedling injury and photosynthetic pigment change in Cucumis sativus and Glycine max. Environ Exp Bot 57:160–167
Acknowledgements
This work was supported by the Bavarian State Ministry of Sciences, Research, and the Arts in the program BayForUV.
Conflict of interest
The authors declare that they have no conflict of interest.
Author information
Authors and Affiliations
Corresponding author
Additional information
Dedicated to Professor Cornelius Lütz on the occasion of his 65th birthday
Rights and permissions
About this article
Cite this article
Götz, M., Albert, A., Stich, S. et al. PAR modulation of the UV-dependent levels of flavonoid metabolites in Arabidopsis thaliana (L.) Heynh. leaf rosettes: cumulative effects after a whole vegetative growth period. Protoplasma 243, 95–103 (2010). https://doi.org/10.1007/s00709-009-0064-5
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00709-009-0064-5