Abstract
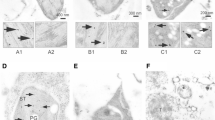
In the light of our previous work, we know that there is a relationship between bound polyamines and the chloroplast differentiation process. This relationship may represent an important component of the process and be part of the mechanism of kinetin action, which stimulates chloroplast differentiation. To clarify the nature of the binding of polyamines to chloroplast structures, the possible involvement of transglutaminases in kinetin-stimulated chloroplast photodevelopment was investigated. Immunodetection of transglutaminases revealed bands at 77, 50 and 30 kDa both in etioplasts and chloroplasts. The data indicated a positive correlation between enzyme level and activity. It also demonstrated the regulation of transglutaminase protein expression by kinetin. The suborganellar location of transglutaminases by electron microscopy showed that the enzyme is peculiarly localised, mainly in pro-thylakoids and appressed grana thylakoids. The data corroborated that spermidine post-translational modification of certain plastid proteins of 58, 29, 26 and 12 kDa occurred. The results we obtained suggest that transglutaminases take part in the formation of the chloroplast structure via a mechanism whereby polyamines bind to their protein substrates. These findings about the effect of kinetin on conjugation provide a new contribution to the understanding of the mechanism of kinetin action on etioplast-to chloroplast transformation.




Similar content being viewed by others
Abbreviations
- LHCPII:
-
light-harvesting chlorophyll a/b complex protein
- PA:
-
polyamine
- PSII:
-
photosystem II
- Put:
-
putrescine
- Spd:
-
spermidine
- Spm:
-
spermine
- TGases:
-
transglutaminases
- DMC, N’:
-
N’-dimethylcasein
References
Andreadakis A, Kotzabasis K (1996) Changes in the biosynthesis and catabolism of polyamines in isolated plastids during chloroplast photodevelopment. J Photochem Photobiol B: Biol 33:163–170
Beigbeder A, Vavadakis M, Navakoudis M, Kotzabasis K (1995) Influence of polyamine inhibitors on light-independent and light-dependent chlorophyll biosynthesis and on the photo-synthetic rate. J Photochem Photobiol B: Biol 28:235–242
Bernet E, Claparols I, Dondini L, Santos MA, Serafini-Fracassini D, Torne JM (1999) Changes in polyamine content, arginine and ornithine decarboxylases and transglutaminase activities during light/dark phases of initial differentiation in maize calluses and their chloroplasts. Plant Physiol Biochem 37:1–11
Brown R, Jarvis K, Hyland K (1989) Protein measurement using bicinchoninic acid: elimination of interfering substances. Anal Biochem 180:136–139
Carvajal-Vallejos PK, Campos A, Fuentes-Prior P, Villalobos E, Almeida AM, Barbera E, Torne JM, Santos M (2007) Purification and in vitro refolding of maize chloroplast transglutaminase over-expressed in Escherichia coli. Biotechnol Lett 29:1255–1262
Del Duca S, Serafini-Fracassini D (1993) Bound polyamines in plants. Curr Top Plant Physiol 1:83–102
Del Duca S, Serafini-Fracassini D (2005) Transglutaminases of higher, lower plants and fungi. In: Mehta K, Eckert R (eds) Transglutaminases. Prog Exp Tum Res. Karger, Basel, pp 223–247
Del Duca S, Favali A, Serafini-Fracassini D, Pedrazzini R (1993) Transglutaminase-like activity during greening and growth of Helianthus tuberosus explants. Protoplasma 174:1–9
Del Duca S, Tidu V, Bassi R, Esposito C, Serafini-Fracassini D (1994) Identification of chlorophyll-a/b proteins as substrates of transglutaminase activity in isolated chloroplasts of Helianthus tuberosus L. Planta 193:283–289
Del Duca S, Dondini L, Della Mea M, Munoz de Rueda P, Serafini-Fracassini D (2000) Factors affecting transglutaminase activity catalysing polyamine conjugation to endogenous substrates in entire chloroplasts. Plant Physiol Biochem 38(6):429–439
Della Mea D, Caparros-Ruiz D, Claparolos I, Serafini-Fracassini D, Rigau J (2004a) AtPng1p. The first plant transglutaminase. Plant Physiol 135:2046–2054
Della Mea M, Di Sandro A, Dondini L, Del Duca S, Vantini F, Bergamini C, Bassi R, Serafini-Fracassini D (2004b) A Zea mays 39 kDa thylakoid transglutaminase catalyses Light Harvesting Complex II by polyamines in a light-dependent way. Planta 219:754–764
Dondini L, Bonazzi S, Serafini-Fracassini D (2000) Recovery of growth capacity by polyamines and of chloroplast transglutaminase activity in a polyamine-deficient variant strain of Dunaliella salina. J Plant Physiol 157:473–480
Dondini L, Del Duca S, Dall’Agata L, Bassi R, Gastaldelli M, Della Mea M, Di Sandro A, Claparos I, Serafini-Fracassini D (2003) Suborganellar localisation and effect of light on Helianthus tuberosus chloroplast transglutaminases and their substrates. Planta 217:84–95
Falcone P, Serafini-Fracassini D, Del Duca S (1993) Comparative studies of transglutaminase-like activity and substrates in different organs of Helianthus tuberosus. J Plant Physiol 142:265–273
Griffin M, Casadio R, Bergamini CM (2002) Transglutaminase: nature’s biological glues. Biochem J 368:377–396
Kotzabasis K (1995) A role for chloroplast-associated polyamines? Bot Acta 109:5–9
Kotzabasis K, Fotinou C, Roubelakis-Angelakis KA, Ghanotakis D (1993) Polyamines in the photosynthetic apparatus. Photosynth Res 38:83–88
Kotzabasis K, Strasser B, Navakoudis E, Senger H, Dörnemann D (1999) Charakterization of photoreceptor(s) responsible for the regulation of the intracellular polyamine level and the putative participation of heterotrimetric G-proteins in the signal transduction chain. J Photochem Photobiol B: Biol 50:45–52
Laemmli UK (1970) Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature 227:680–685
Legocka J, Żarnowska A (1999) Role of polyamines in the cytokinin-dependent physiological processes. I. Effect of benzyladenine on polyamine during chloroplast differentiation in the tissue culture of Dianthus caryophyllus. Acta Physiol Plant 21:349–354
Legocka J, Żarnowska A (2000) Role of polyamines in the cytokinin-dependent physiological processes. II Modulation of polyamine levels during cytokinin-stimulated expansion of cucumber cotyledons. Acta Physiol Plant 22:395–401
Legocka J, Żarnowska A (2002) Role of polyamines in the cytokinin-dependent physiological processes. III Changes in polyamine levels during cytokinin-induced formation of gametophore buds in Ceratodon purpureus. Acta Physiol Plant 24:303–309
Lilley G, Skill J, Griffin M, Bonner P (1998) Detection of Ca2+-dependent transglutaminase activity in root and leaf tissue of monocotyledonous and dicotyledonous plants. Plant Physiol 117:1115–1123
Lorand L, Graham RM (2003) Transglutaminases: crosslinking enzymes with pleiotropic functions. Nature Rev Mol Cell Biol 4:140–156
Margosiak SA, Dharma A, Bruce-Caver MR, Gonzales AP, Louie D, Kuehn GD (1990) Identification of the large subunit of ribulose 1,5-bisphosphate carboxylase/oxygenase as a substrate for transglutaminase in Medicago sativa L. (alfalfa). Plant Physiol 92:88–96
Parthier B (1979) The role of phytohormones (cytokinins) in chloroplast development. Biochem Physiol Pflanz 174:173–214
Signorini M, Beninati S, Bergamini C (1991) Identification of transglutaminase activity in the leaves of silver beet (Beta vulgaris L.). J Plant Physiol 137:547–552
Slaughter TE, Komandor AE, Lai TS, Greenberg CS (1992) A microtiter plate transglutaminase assay utilising 5-(biotinamido)pentylamine as substrate. Annal Biochem 205:167–171
Sobieszczuk-Nowicka E, Di Sandro A, Del Duca S, Serafini-Fracassini D, Legocka J (2007a) Plastid-membrane-associated polyamines and thylakoid transglutaminases during etioplast-to-chloroplast transformation stimulated by kinetin. Physiol Plant 130:590–600
Sobieszczuk-Nowicka E, Rorat T, Legocka J (2007b) Polyamine metabolism and S-adenosylmethionine decarboxylase gene expression during the cytokin-stimulated greening process. Acta Physiol Plant 29:595–502
Villalobos JM, Torne J, Rigau I, Olles I, Claparos M, Santos V (2001) Immunogold localisation of transglutaminase related to grana development in different maize cell types. Protoplasma 216:155–163
Villalobos E, Santos M, Talavera D, Rodrıguez-Falcon M, Torne JM (2004) Molecular cloning and characterization of a maize transglutaminase complementary DNA. Gene 336:93–104
Acknowledgements
The author is very indebted to Prof. Donatella Serafini-Fracassini and her co-workers from Bologna University for methodological support, to Agnieszka Kazikowska for the processing of the figures and to Heidi Nicholl of City University, London for the linguistic correction of the manuscript.
This work was partly supported by the Polish State Committee for Scientific Research grant number 2 PO4C 085 26.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Sobieszczuk-Nowicka, E., Krzesłowska, M. & Legocka, J. Transglutaminases and their substrates in kinetin-stimulated etioplast-to-chloroplast transformation in cucumber cotyledons. Protoplasma 233, 187–194 (2008). https://doi.org/10.1007/s00709-008-0002-y
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00709-008-0002-y