Summary.
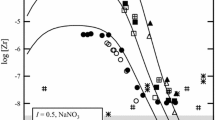
Solubility measurements as a function of temperature have been shown to be a powerful tool for the determination of thermodynamic properties of sparingly-soluble transition metal carbonates. In contrast to calorimetric methods, such as solution calorimetry or drop calorimetry, the evaluation of solubility data avoids many systematic errors, yielding the enthalpy of solution at 298.15 K with an estimated uncertainty of ±3 kJ · mol−1. A comprehensive set of thermodynamic data for otavite (CdCO3), smithsonite (ZnCO3), hydrozincite (Zn5(OH)6(CO3)2), malachite (Cu2(OH)2CO3), azurite (Cu3(OH)2(CO3)2), and siderite (FeCO3) was derived. Literature values for the standard enthalpy of formation of malachite and azurite were disproved by these solubility experiments, and revised values are recommended. In the case of siderite, data for the standard enthalpy of formation given by various data bases deviate from each other by more than 10 kJ · mol−1 which can be attributed to a discrepancy in the auxiliary data for the Fe2+ ion. A critical evaluation of solubility data from various literature sources results in an optimized value for the standard enthalpy of formation for siderite. The Davies approximation, the specific ion-interaction theory, and the Pitzer concept are used for the extrapolation of the solubility constants to zero ionic strength in order to obtain standard thermodynamic properties valid at infinite dilution, T = 298.15 K, and p = 105 Pa. The application of these electrolyte models to both homogeneous and heterogeneous (solid-solute) equilibria in aqueous solution is reviewed.
Similar content being viewed by others
Author information
Authors and Affiliations
Additional information
Received June 26, 2001. Accepted July 2, 2001
Rights and permissions
About this article
Cite this article
Preis, W., Gamsjäger, H. Thermodynamic Investigation of Phase Equilibria in Metal Carbonate–Water–Carbon Dioxide Systems. Monatshefte fuer Chemie 132, 1327–1346 (2001). https://doi.org/10.1007/s007060170020
Issue Date:
DOI: https://doi.org/10.1007/s007060170020