Abstract
Since the industrial revolution the world economy has grown thanks to energy generated by cheap and abundant fossil resources. Today, due to climate change caused by human-induced CO2 and other greenhouse gas emissions, we are in the midst of a shift away from fossil fuels and towards renewable energy. It remains to be seen how fast this shift will happen and whether the correlating economics are feasible. Increasingly, experts are asking: what role will catalysis play to help drive energy efficiency? With a 60-year history in industrial catalysis, Clariant’s Catalysts business has been a front runner in catalyst and technology development. For more than a decade we have been working in the catalytic Power-to-X segments. This paper presents our understanding on current developments around the important role of Power-to-X and Waste-to-X technologies to enable a worldwide transition of the energy sector to renewables.
Graphical abstract

Similar content being viewed by others
Introduction
For decades the major equation ruling the energy industry has been the following: take cheap abundant fossil resources—that is, coal, oil, and gas—convert them with oxygen to generate steam to produce electricity and sell it at attractive price levels to fuel the world’s societies.
Today, based on the detrimental impacts of global warming caused by carbon dioxide (CO2) and other greenhouse gases released from fossil resources, the urgency to quit the world’s current emission path is more than obvious. Yet, a fundamental question remains: can the old energy equation, as well as the correlating economics, be reversed? Will it be possible to provide the world’s future and growing energy needs with green electricity from renewable sources together with green energy based on biomass?
Increasingly, industry experts are also asking: what role will catalysis play in the production of chemicals and fuels? That is because catalysis, by its nature, has always been a means to achieve improved energy efficiency. For example, in ammonia production, energy efficiency has risen substantially over the last 100 years, resulting in a reduction of the energy demand to produce 1 ton of ammonia from 400 gigajoules to below 50 gigajoules [1].
While further efficiency improvements in industrial catalytic processes are ongoing, via the aligned development of catalysts and process technology, it's fair to say that all the low hanging fruits have been picked. However, there is a new exciting and major target in catalysis research and development to provide catalytic materials and processes for the conversion of renewable hydrogen (H2) with various reaction partners to green energy carriers, as a substitute for fossil oil and gas. The current understanding is that the future import level of such renewable energy carriers to some european countries such as Germany will be more than 80%, very similar to the current one of fossil energies.
Clariant’s role as a globally leading producer of high-performance catalysts
As one of the world’s leading specialty chemical companies, Clariant contributes to value creation with innovative and sustainable solutions for customers from many industries. Our portfolio is designed to meet very specific needs with as much precision as possible. At the same time, our research and development are focused on addressing the key trends of our time. These include energy efficiency, renewable raw materials, emission-free mobility, and conserving finite resources.
Building on a 165-year history, Clariant Catalysts is a global and independent manufacturer of high-performance, energy-efficient catalyst technologies shaping a sustainable future for the chemical industry, helping to defossilize customers’ production processes, and scaling up the transition toward zero-emission chemicals and fuels. Clariant Catalysts is made up of global teams of approximately 2,000 employees in 10 research & development (R&D) and technical centers and 15 production sites. Thanks to its broad know-how and materials portfolio in the syngas and refinery sector, Clariant Catalysts is an ideal partner for technology developers who do not produce catalysts themselves.
An introduction to Power-to-X technology for the energy transition
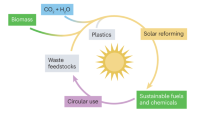
Power-to-X (P2X, Fig. 1) is a broad technology field but essentially means converting power into carbon–neutral, chemical energy carriers. In a first step, renewable electricity, via electrolysis of water, is converted to renewable H2 and subsequently through catalytic conversion (with the reaction partners CO2, nitrogen (N2), or organic molecules) to gaseous or liquid energy carriers, which, compared to H2, are easier and cheaper to store and transport.
Due to their significantly reduced CO2 footprint, compared to fossil fuels, P2X energy carriers will help to reduce carbon emissions in all sectors of the energy system: from electricity, heating, and industry to mobility. In mobility, P2X fuels should not be seen as an alternative to e-mobility but rather as a useful complement in areas where e-mobility is not a good choice such as in heavy road transport, shipping, or aviation.
Besides conversion the other very important contribution of P2X and Waste-to-X (W2X) is to supply suitable economic materials and concepts to purify CO/CO2, as, depending on the CO/CO2 source, it may come with a variety of impurities that might poison the downstream synthesis catalysts.
Motivation
Human-induced climate change is causing dangerous and widespread disruption in nature and affecting the lives of billions of people around the world, despite efforts to reduce the risks. A report from the IPCC published in 2022 [2] found that the world faces unavoidable multiple climate hazards over the next two decades with global warming of 1.5 °C and that even only temporarily exceeding this warming level will result in additional severe impacts, some of which will be irreversible.
Yet, carbon emissions continue to rise. Figure 2 shows that since 1993, in just one generation, emissions of CO2 into the atmosphere have doubled [3].
To avoid mounting loss of life, biodiversity, and infrastructure, ambitious, accelerated action is required to adapt to climate change, at the same time as making rapid, deep cuts in greenhouse gas emissions. It is clear there is an urgency to develop low-carbon technologies using approaches such as Power-to-X and Waste-to-X.
Clean hydrogen technologies as a prerequisite for Power-to-X
All Power-to-X (P2X) technologies rely on the availability of large amounts of clean H2, another sector where research is accelerating with unprecedented momentum.
Drivers for clean hydrogen
To a large extent the push towards clean H2 is being driven by upcoming regulations and laws, particularly in the European Union, which has set itself the ambitious goal to be the first carbon neutral continent by 2050. To achieve carbon neutrality in this timeframe, the European Green Deal has set an interim CO2-reduction target of 55% by 2030 [4], meaning a necessary revision of all relevant climate-related policy instruments.
Published in May 2022, the REPowerEU plan [5] completed the implementation of the European hydrogen strategy, further increasing the European ambitions for renewable H2 as an important energy carrier to move away from fossil fuel imports. It aims to produce 10 million tons and import 10 million tons of renewable H2 into the EU by 2030 — a substantial increase from the 5.6 million tons foreseen within the revised Renewable Energy Directive [6], published in July 2021. Many of the European member states have included clean H2 plans in their national policy frameworks [7].
Triggered by this policy framework European players in the fossil-based H2 economy have joined forces in strategic alliances like the Hydrogen Council or the European Clean Hydrogen Alliance, to install 80 gigawatt (GW) of electrolysis by 2030, with the development of a clear roadmap of viable investment projects. These 80 GW roughly correlate to the necessary installed capacity to provide 10 Mio tons of hydrogen with an energy content that corresponds to 45% of the energy supplied to the mobility sector in Germany (2019) by fossil fuels, diesel, kerosene, and gasoline [8].
As the cost of renewable H2 production depends on investment cost in electrolysis and power, the other important driver of current green H2 and P2X momentum is the cost decline of solar and wind power by around 80 percent over the past decade. This cost decline is reflected in the IEA’s Renewables Report 2020, which outlines that total installed wind and photovoltaic capacity is on course to surpass the capacity of natural-gas-based electricity generation in 2023, and that from coal in 2024, to become the largest source of electricity around 2025 [9].
Production cost of clean hydrogen
Today, the business case for P2X remains weak because green H2 is still too expensive (Fig. 3). However, based on the scale of currently announced green H2 projects worldwide, which in 2022 added to more than 380 GW, economy of scale is expected to efficiently bring down investment costs. It is estimated that green H2 will achieve cost parity with fossil H2 around 2030 [10].
Somewhat different to H2 production from fossil natural gas, renewable H2 plants must take a high initial investment hurdle (CAPEX) to provide green electricity via installations of photovoltaic or wind parks upstream of the electrolysis plant. The European Commission estimates that the necessary investment for the above-mentioned 80 GW electrolysis will range up to €42 billion, in addition another €220–340 billion to install 80–120 GW capacity of solar and wind will be needed, as well as another ~ €65 billion in means for H2 transport, distribution, and storage [11].
On the other hand, after amortization, operating costs (OPEX) over the remaining lifetime of renewable H2 plants will be low as the “input fuels”—the wind and the sun do not send bills. Assuming that the taxation of CO2 emissions will continue to increase worldwide, renewable H2 may represent the better solution from both an environmental and economic perspective on the longer term.
In the short and medium term, the clear priority for the EU is to develop renewable H2, from wind, hydro and solar energy, however, other forms of low-carbon H2 will be needed to rapidly reduce emissions from existing H2 production and to support the technology rollout of renewable downstream technologies, such as CO2/H2 conversion to SynFuels.
Amongst these clean H2 technologies, blue H2, produced from natural gas and supported by carbon capture and storage (CCS), is already close to cost parity, as existing assets to produce fossil H2 via steam reforming of natural gas can be used. This is today’s mature industrial technology for H2 production, however blue H2 scale-up requires the high techno–economic maturity of CCS technology, to permanently store the CO2 generated underground.
In the longer term, the growth potential for clean H2 seems huge [12]: Depending on different scenarios to achieve the well below 2 °C Paris goal, low-carbon H2 will be required at a scale which contributes to ~ 20% of the world’s energy use in 2050. This correlates to an installed capacity of green and blue H2 of around 5 Terawatt, similar to the currently installed global capacity to supply electricity from coal.
Clariant contributions to provide clean hydrogen
Regarding blue H2, Clariant has already adapted its existing portfolio of reforming and water–gas shift catalysts to supply blue H2 (H2 from natural gas) to enable higher energy efficiency and higher CO2 concentrations to improve the techno-economics for the subsequent underground storage of CO2.
In so-called Waste-to-X technologies, to provide green H2 from biomass or from biogenic residues, Clariant supplies a large portfolio of sorbents and catalysts which are mandatory for gas purification and gas conditioning. In green H2 production from electrolysis, Clariant supplies catalysts to remove impurities from electrolytic H2, such as highly efficient Platinum (Pt) or Palladium (Pd) based catalysts for the removal of oxygen, which could otherwise poison the catalysts for downstream catalytic conversion to renewable energy carriers such as green methanol.
Once H2 is available in sufficient purity, Clariant catalysts and technologies for H2 logistics, such as long-distance transport via ammonia or Liquid Organic Hydrogen Carriers (LOHCs), kick in.
Clariant technologies for hydrogen storage and transport
Storage & transport of hydrogen via LOHC
Clariant began catalyst development for Liquid Organic Hydrogen Carriers around 2010, in close cooperation with Prof. Peter Wasserscheid, Lehrstuhl für Chemische Reaktionstechnik at the Friedrich-Alexander-University in Erlangen.
The underlying concept of the LOHC approach [13] is that H2 is transferred to an aromatic type of liquid organic carrier, such as benzyl toluene, in an exothermic reaction using suitable hydrogenation catalysts, which results in a saturated hydrocarbon. Later, from the saturated hydrocarbon, H2 can be released by suitable dehydrogenation catalysts, based on Pt or Pd, in an endothermic reaction depending on the actual demand of H2.
The first big advantage of this concept is the high energy density of the hydrogenated hydrocarbon carrier, which is comparable to methane at 200 bar or H2 at 700 bar, but much easier to store or transport, as it is liquid at ambient condition, at 1 bar and RT. The other is its high intrinsic safety, as H2 release only occurs in contact with the catalyst at sufficiently high temperatures.
Clariant’s cooperation with the chair of Chemische Reaktionstechnik was very successful and, in 2013, Hydrogenious LOHC Technologies was founded as a spin off from the university, to bring the technology for very safe, easy, and flexible H2 storage and transport to the market, providing industrial-scale LOHC (de)hydrogenation systems as key components for connecting H2 producers and off takers.
Currently Hydrogenious is pushing long-distance clean H2 exports/imports worldwide, with a focus on establishing supply chains between the Middle East and Germany. Among others, jointly with ADNOC, JERA Americas and Uniper, the company is exploring a large-volume and low-carbon H2 supply chain from the United Arab Emirates to Europe. The project includes the development of a commercial demonstration project with LOHC plant systems from Hydrogenious and the associated infrastructures with volumes of approx. 10,000 up to 180,000 tons of H2 per year.
Power to synthetic natural gas (SNG)
In the future, once fossil resources are rare, the use of CO2 as a feedstock for the synthesis of synthetic natural gas (SNG) or methanol, is not a new idea. The most prominent protagonists suggesting the approach were Friedrich Asinger [14] as early as 1986, and the Nobel Laureate Georg Olah in 2005 [15], who postulated the often-cited methanol economy due to the high versatility of methanol as a base chemical and fuel, and its potential to prevent global warming once produced from renewable H2.
Years later Michael Sterner [16] in cooperation with Michael Specht from the ZSW, Germany’s Center for Solar Energy and Hydrogen Research, developed the idea of “sector coupling”. In this approach renewable electricity is first converted via electrolysis to H2, which is then catalytically converted with CO2 to Synthetic Natural Gas, a reaction first investigated in 1902 by Sabatier and Senderens [17].
Around 2012, Clariant started to develop catalysts for CO2/H2 conversion, in cooperation with ZSW, MAN, Audi, and EtoGas and developed a methanation catalyst for the world ‘s first Power-to-SNG plant in Werlte, Germany to supply synthetic natural gas for Audi ‘s renewable mobility concept.
In this process, CO2 from a biogas plant and H2 from electrolysis were reacted in a MAN reactor concept, equipped with efficient means for cooling, then filled with a Clariant nickel-based catalyst. To defossilize the mobility sector this is a clever concept as compressed synthetic natural gas is an efficient and very clean burning fuel, with both low nitrogen oxides (NOx) and particulate emissions.
Logistics in Germany and other European countries are very suitable for this approach as well. Germany’s natural gas grid, with its high capacity, could be used for storage and transport. However, in 2020 Audi sold the P2X plant in Werlte. As a spin off it is now operated by a new, independent company.
However, since then Clariant’s technology partner MAN, in anticipation of a more suitable, European regulative framework, has further developed the technology based on Clariant catalysts, and now offers a modular 50 Megawatt (MW) plant concept, integrating CO2 separation, electrolysis and catalytic conversion. Currently, MAN and Clariant are preparing to upscale and market the SNG technology for the so-called Nordur project [18], which aims to produce SNG, based on the abundant geothermal energy in Iceland, and to export it via ship to Basel, where the renewable SNG may then be fed into the Swiss gas grid to defossilize heating energy for Swiss households.
As approximately 40% [19] of global-CO2 emissions are generated by the heating sector, the injection of renewable SNG to existing gas grids is an innovative way to reduce the carbon footprint of heating in the private and industrial sectors. Furthermore, in several European member states feed-in tariffs for renewable green gas have been introduced [20].
Power-to-ammonia
An approach that entered the Power-to-X stage somewhat later, but that has gained huge momentum in the recent years, is Power-to-ammonia.
Of the earlier outlined 380 GW of planned green H2 projects worldwide, the largest share of developers with defined H2 carriers have currently opted for ammonia, with about 35% is set to convert green H2 to green ammonia. One key rationale is that many regions with large renewables potential are remote, but that is where the large-scale green H2 projects in the Gigawatt range are being developed, such as in Saudi-Arabia, Western Australia, and Chile.
Since H2 transport is expensive, and comes with significant energy loss, either for compression or liquefaction, catalytic conversion to ammonia is seen to be better suited to support the growth of a worldwide market to trade clean H2 between supply and offtake states.
As today already 20 Mio tons of ammonia are transported by sea each year, it is considered as the low hanging fruit for long-distance H2 transport. Clariant is well established to participate in this growth market, with its state-of-the-art AmoMax® series iron-based wüstite catalysts, that are also applicable for green ammonia production. In 2020, Clariant and its technology partner Casale won the prestigious ICIS award for the highly efficient ammonia synthesis technology [21] they developed.
Green ammonia has several direct use cases:
-
It can be used as a marine fuel in shipping
-
Japan is aiming to reduce emissions from its coal-based power plants by the co-firing of green ammonia
-
And the most important role of green ammonia for the decades to come will be to defossilize the rising volume of fertilizer production necessary to nourish an ever-growing world population.
In its function as a carrier molecule for H2, and to supply H2 to the growing fuel cell market, technology for ammonia decomposition is also required. Together with technology partners Clariant is actively developing more efficient new versions for its ammonia cracking catalysts HyProGen® 800 DCARB series.
Power to methanol
The probably most important pillar in P2X and W2X technologies is methanol, a major and highly versatile bulk chemical with a production volume of ~ 100 Mio tons year.
Established methanol synthesis technologies and market
With regard to industrial synthesis of methanol, similar progress like for ammonia was made: with the development of a copper–zinc catalyst by Imperial Chemical Industries in the 1960, to replace the early high-pressure methanol process developed by BASF in the 1920s, a significant reduction of reaction pressure and temperature was achieved while syngas conversion to methanol could be more than doubled.
A comprehensive description of current and future expected production volumes as well as the use of methanol in different sectors and applications can be found in the International Renewable Energy Agency (IRENA)’s Innovation Outlook on renewable methanol [22].
According to that report methanol production since 2010 has grown significantly at close to 100 Mt in 2019, driven by growing demand and production volumes based on coal in China, and could grow to 500 Mt in 2050.
Established fossil processes for methanol production, such as combined reforming, are based on natural gas. Combined reforming is a complex process chain starting with a CO2-intensive reforming step, with part of the natural gas burned to supply the high reaction temperature that is needed to reform the other part of the natural gas with steam and oxygen to produce syngas with a high CO content and high productivity in the catalytic conversion. The resulting CO2 emissions from that process chain are ~ 1.5 tons CO2 to produce 1 ton of methanol [23] (Fig. 4).
Significantly higher CO2 emissions of 3–5 tons CO2 per ton methanol produced, arise, if methanol is produced from coal, with the gasification process and the water–gas shift reaction as the main emission sources [24].
With two thirds of the 98 Mt methanol being produced from natural gas and one third from coal (IRENA&MI), methanol as of today accounts for approximately 0.7% of man-made CO2 emissions (own calculation).
Besides to the production of other essential base chemicals or plastics, about one third of today’s methanol use is in the marine and road transport sector with different options to apply methanol: it can be pure, blended with gasoline in the form of dimethyl ether, or a component in biodiesel or in methyl tert-butyl ether [25].
Today less than 0.5% of methanol is renewable, either produced based on syngas from the reforming of biogas or from the gasification of biomass, municipal waste, or other waste streams, in a small number of plants [26].
Methanol synthesis from carbon dioxide and hydrogen
Higher potential to produce renewable methanol is expected from conversion of pure CO2—respectively CO2-rich syngas feeds with renewable H2 and has the potential to reduce the CO2 emission to < 0.2 tons CO2 / ton methanol [27].
In the early 1990’s Lurgi demonstrated this feasibility based on a new copper–zinc oxide catalyst developed by Süd-Chemie at that time [28].
Looking at the analogies between classic CO and CO2 hydrogenation the suitability of Copper Zinc Alumina (CuZnAl) based catalysts may not be too surprising.
while the syntheses of methanol from CO and CO2 are tied through the WGS reaction:
The key difference is easy to point out: the higher the CO2 content in the feed, the higher the amount of product water, which means a potential threat for the catalyst’s hydrothermal stability.
For now methanol from CO2 and green H2 is being produced at a low scale (4000 tons/yr.) only, at a pilot plant in Iceland. However, larger plants are under construction and in 2024 the world’s largest e-methanol plant, built by European Energy in Denmark, will be started up with Clariant’s MegaMax catalyst.
Carbon2Chem (C2C)
To understand the performance of Clariant’s MegaMax® CuZnAl-based catalysts, Clariant partnered with a large German initiative called Carbon2Chem. It began in 2016 as a consortium of academic and industrial partners to develop technologies for the conversion of steel off-gases, from a thyssenkrupp steel plant in Duisburg, to produce various base chemicals with focus on methanol and ammonia.
The major goal of C2C is to develop the gas purification and subsequent catalytic conversion technology, to utilize the huge gas stream from the blast furnace, and to identify the best strategy for large-scale methanol synthesis from the techno–economic point of view. This is a quite challenging task as depending on the investigated scenarios, the syngas is either rich in CO2 or brings a large share of inert N2 contained in the blast furnace off-gas.
For these scenarios Clariant’s MegaMax® 800 industrial catalyst was intensively evaluated, including intermittent process conditions, which did not negatively affect the catalyst performance, nor its stability as validated by Fraunhofer ISE during miniplant long time testing over more than 2300 h [29].
In addition, to evaluate the requirements for gas purification prior to catalytic conversion, the consortium worked hard to understand the influence of impurities contained in the steel off-gas, as it could potentially affect the performance of the synthesis catalyst.
Results have been described by various members of the C2C consortium, including the academic partners at the Ruhr University Bochum, Fraunhofer ISE, Fraunhofer UMSICHT and the Max-Planck Institute for Chemical Energy Conversion, in various articles and three full CIT (Chemie Ingenieur Technik) booklets.
These investigations identified and divided impurities into two categories: reversible impurities that reduce methanol productivity while present in the feed gas stream, but where productivity recovers once they are removed from the feed, versus irreversible impurities, where productivity remains at a lower level, e.g., for sulfur compounds, which chemically react with the active catalytic sites of the catalyst.
The studies showed that a major precondition for the synthesis of renewable methanol from steel off-gas, next to the availability of large amounts of green H2, is purification to clean the steel off-gases from those contaminants.
Figure 5 shows the gas cleaning plant established at the steel plant in Duisburg. The plant operation is based on Clariant’s gas purification / conditioning catalysts and sorbents and the results achieved so far look very promising, with levels of sulfur and other relevant contaminants in the off gas from the blast furnace reduced to the 1-digit ppb level. Subsequent methanol synthesis, based on Clariant’s MegaMax® 800 catalyst proved very robust and productive.
A big advantage of the development work performed in C2C, is that the know-how and process technology will be transferable to a large extent to other CO2 intensive industries, such as cement production and waste incineration as well.
Green methanol from biogenic CO2
Next to CO2 from large fossil point sources also biogenic CO2 arising from fermentation processes can be used, from smaller plants in a more decentralized approach. This demonstration was undertaken by Clariant in cooperation with thyssenkrupp Industrial Solutions, Südzucker, and Fraunhofer ISE, to demonstrate the feasibility of methanol coproduction from two bioethanol plants. The first was a Südzucker first generation bioethanol plant in Zeitz, Germany, the second was Clariant’s second generation Sunliquid demonstration plant in Straubing, which is based on straw residues as the raw material for fermentation.
One advantage is a reduced effort for purification, as CO2 from fermentation processes, such as bioethanol or biogas production, comes in high concentrations and is already quite pure. An important scientific result of the project was produced by academic partners of Fraunhofer ISE by means of an improved kinetic model for methanol synthesis from CO2-rich synthesis gases [30]. In addition, the result of a life cycle analysis, as a major key performance indicator for the potential to reduce CO2 emissions, will most probably be more favorable in the light of upcoming regulations, than for the case of fossil CO2 use.
Improved methanol synthesis catalyst for CO2-to-methanol
In the above-mentioned demonstrations, Clariant’s standard MegaMax® 800 catalyst proved its high performance. However, there is always room for improvement, so Clariant optimized the quite complex composition with a focus on its hydrothermal stability. This resulted in a new catalyst lead with significantly increased stability and productivity under CO2-to-MeOH conditions, that outperforms previous generations and MegaMax® 800 as a benchmark. This new product, MegaMax® 900 DCARB, will be available in 2024.
Alternative catalysts
With the rising interest in CO2 conversion technologies over the last decade, several alternative catalysts with promising potential have been described in academia and include modifications of the ternary Cu/ZnO/Al2O3 catalysts with zirconia phases, promotion of copper with gallium new active mass catalysts like Pd on different oxidic supports, and ZrO2-supported In2O3. Despite these, and other, strong academic efforts and successes, as of today the ternary Cu/ZnO/Al2O3 system remains the benchmark standard that needs to be met when it comes to industrial applicability like productivity, lifetime, and cost of ownership.
CO2-to-methanol economics
Technically CO2-to-methanol technology is proven and available at a high technical readiness level, but what about the economics? Figure 6 is the result of a detailed analysis performed by Clariant to better understand the specific methanol production cost for a large-scale combined electrolysis & methanol plant, which is, for the most part, dominated by the production cost of green H2.
The important take away from this analysis is that to bring down costs, such a plant has to operate at the minimum cost of electricity and maximum time-on-stream, according to the best-case scenario (Number 3). In this scenario production costs are still higher than for fossil methanol, but comparable with the current sales price in Europe and the production cost of other advanced biofuels such as hydrogenated fatty acids or hydrogenated vegetable oils.
This result is in qualitative accordance with that of other groups [31]. In addition, any future CO2 taxation will further underpin the economics to produce renewable methanol. If we assumed that the result of a certified life cycle analysis confirms a reduction in CO2 emissions of 1.3 tons for methanol produced from CO2 and green H2 compared to fossil methanol from natural gas, and by applying a carbon credit at 100 €/ton, the economic advantage would result in cheaper methanol at 130 €/ton [32].
Compared to other renewable energy carriers such as H2, liquified natural gas, or ammonia, methanol has one big advantage—it can be transported long distances without the need for pressurization or cooling while its energy density at ambient temperatures is significantly higher than pressurized or liquified H2 or liquified ammonia [33]. In principle, that would make renewable methanol imported from remote areas with large renewable potential quite attractive. For the time being, however, logistics will be more demanding to supply the CO2 reaction partner e.g., transport by ship, as the cost of CO2 separation at the plant site from air are too high and significantly higher than to supply the reaction partner N2 for ammonia synthesis.
Adding an additional step in the value chain, the further conversion of methanol to gasoline is another interesting option currently being explored by a consortium in Chile at the Haru Oni demonstration plant. Led by HIF (Highly Innovate Fuels) [34] and supported by the energy partnership Chile-Alemania, it has prominent partners including Siemens to supply the electrolysis technology and Porsche as fuel off-taker.
Power-to-Methanol and Power-to-Liquids to defossilize aviation and shipping
Regulations
Within upcoming European legislation, aviation and shipping are the sectors in transport which face the highest regulatory pressure to reduce their emissions. Both sectors, similar to the steel or cement sector, contribute ~ 2–3% of global-CO2 emissions each.
In 2021/2022, in the course of the so-called Fit for 55 package [35] (Fig. 7) several new initiatives to reduce emissions have been introduced by the European Commission, including the ReFuelEU Aviation and the FuelEU Maritime Initiatives, both in the form of legally binding regulations.
The Aviation Initiative [36] aims to boost the supply and demand for sustainable aviation fuels in Europe. Current draft regulations propose obligations on fuel suppliers to increasingly distribute/blend sustainable aviation fuels over time. It is expected that advanced biofuels will provide the highest volumes as indicated by the percentage numbers in green. In addition, the blue numbers show a sub-mandate for so-called Renewable Fuels of Non-Biological Origin—a synonym for P2X based fuels, also called e-fuels — that will also be in place.
The Maritime initiative is set up a little differently. Instead of a requirement to increase the blending of e-fuels over time, there will be an obligation to gradually decrease the greenhouse gas emissions intensity of energy used onboard ships.
Methanol to defossilize the shipping sector
In 2022, Maersk, one of the world’s leading operators of ocean carriers, announced that it would discontinue the use of fossil fuels to power the company’s container vessels and explained that it had ordered the construction of 19 new large vessels (status at the end of 2022) which are able to run on green methanol.
Such announcements may indicate a breakthrough for green methanol producers, as now methanol off takers with a strong-regulative need are in place, like Maersk, which now is actively evaluating where they can source a corresponding amount of ~ 750.000 tons green methanol/year, which is, yet, not available.
With more than 5000 large vessels on the high seas, transporting 80% of all goods traded worldwide, there is large potential for green ammonia and green methanol to defossilize the shipping sector.
Clariant’s contribution to Power-to-Liquids to defossilize long-distance aviation
In addition to the direct use of green methanol in shipping, it will also serve as an important building block to provide renewable kerosene via the so-called Methanol to Jet approach, which is based on one of Clariant’s core areas of expertise—the zeolite catalysis platform.
In this, MFI type zeolites are used to first convert methanol to a blend of lower olefines in the range of three to six carbon atoms. In a second step these olefines are further oligomerized and hydrogenated to middle distillates with a maximized slate of kerosene.
In 2022 various consortia [37] worldwide, including big players such as ExxonMobil, announced demonstration projects using this highly attractive route to achieve the necessary ASTM specification, which will allow the blending of the methanol-based product with conventional kerosene. Clariant is one partner in the “SAFari” consortium, announced in early 2023, bringing its know-how and catalysts in zeolite-based acid catalysis.
The alternative renewable pathway to produce synthetic kerosene is the Fischer Tropsch route. One advantage is that it has already been used in successful demonstration projects in a so-called Waste-to-X approach, based on the conversion of syngas from the gasification of biomass. Complex ASTM certification has been achieved up to a blending ratio of 1:1 with conventional kerosene.
Following the Fischer–Tropsch route from CO2 and renewable H2, using the P2X approach, a reverse water–gas shift (RWGS) reaction step is needed upstream to supply syngas which is rich in carbon monoxide (CO) via RWGS:
This step is required as the efficient cobalt-based catalysts that are widely applied for low-temperature Fischer–Tropsch synthesis, are not suitable for directly converting CO2.
In 2022 a first modular industrial-scale production plant for P2X based Fischer–Tropsch kerosene technology was started by Ineratec together with atmosfair, a non-profit organization. Ineratec is the technology provider for the synthesis technology.
Clariant and Ineratec joined forces several years ago to progress the application of Clariant’s catalyst in Ineratec’s highly innovative chemical reactors. The syngas reactor uses a promoted nickel Clariant catalyst with high resistance against coking, high CO2 conversion and low methane by-product formation. It was already successfully applied to supply the CO rich synthesis gas via the so-called reverse water gas shift (RWGS) reaction step in this project as well as in several other projects.
Next scale industrial and commercial projects are currently in preparation based on this catalyst. Ineratec, together with Clariant and partners, is realizing a P2X production plant with the focus on synthetic kerosene at the InfraServ Industriepark in Frankfurt Höchst [38], with the target to start operation in 2023.
In December 2022, the UK Government Department for Transport granted an award of £2.5 million to a Velocys e-fuels project (known as ‘e-Alto’). The project will be supported by partners, which include British Airways, Technip Energies, and Clariant Catalysts. The e-Alto Sustainable Aviation Fuel (SAF) project is intended to demonstrate Fischer–Tropsch-based SAF production from CO2 and H2 in the UK. Again, the development of an efficient, first RWGS technology step to supply syngas will be based on the Clariant catalyst and know-how.
Sources for carbon dioxide
As carbon-based Power-to-X technologies rely on CO2 as a raw material, a key question arises—which CO2 source is the most appropriate?
There are frequent discussions on where the CO2 raw material should be sourced once the approximately 19 Gigatons of CO2 emissions from today’s big fossil point sources, such as power plants from the oil and gas industry, or from the cement or steel production, are hopefully rapidly and dramatically reduced. In this context, what needs to be understood is that, even for a very optimistic well-below 2 °C global warming scenario, in 2040 more than > 7 Gigatons of CO2 emissions from big industrial point sources will still be available [39] (Fig. 8).
Let us undertake a short thought experiment focused on that 7 Gt scale of CO2 emissions: Assuming sufficient green H2 is available as a reaction partner, more than 4500 Mio tons of green methanol could be produced, more than 40-fold the volume of current world methanol production.
Thinking decades ahead, technologies to extract CO2 from the atmosphere could be available. Today, Direct Air Capture (DAC) is positioned as the most sustainable source for CO2, however this needs more differentiated understanding: as long as unavoidable fossil CO2 emissions remain an economic reality, it is reasonable to ask why CO2 would not be captured in high concentration at big point sources rather than let CO2 diffuse into the atmosphere to be captured from there later, in very low concentrations at a significantly higher cost and energy penalty.
This should not be understood as an argument against DAC, as it will be needed long-term as a technology to achieve negative emissions, in order to balance the surplus of emissions, that we will emit beyond the remaining carbon emission budget (1000 Gigatons to stay in line with the 2.0 °C goal, or 400 Gigatons to stay in line with 1.5 °C global warming [40]).
Summary
It is obvious that green hydrogen from electrolysis will be the central element in establishing a future energy system based on renewables, as demonstrated by the exponential growth in the clean hydrogen market in the last 2 and 3 years. To a large extent this is being driven by net zero ambitions and upcoming regulations in the EU and worldwide.
Consequently, the cost of green hydrogen is expected to come down efficiently, achieving cost parity with fossil hydrogen production in renewable sweet spots of the world around 2030. For many of those regions, the availability of catalytic P2X technologies and energy carriers offers a new and very attractive business model to export their renewable potential to countries like Germany, that have less-renewable potential but have great ambitions to reduce their emissions.
For energy, technology, and material providers as well, there are huge economic opportunities but also challenges ahead in financing and installing the necessary new technology and infrastructure. A major task in technology development is in the reduction of energy losses across the P2X process chain, to convert a maximum share of the primary electrical energy from wind and photovoltaics to the different P2X energy carriers.
As of today, the economics are not yet competitive, and effective government support schemes are crucial to bridge the existing economic gap. These should include instruments such as financial guarantee mechanisms for larger pilot projects or long-term offtake contracts to create security for the high initial investments needed to be made for the entire Power-to-X value chain.
In that context, next to green hydrogen, blue hydrogen will be needed to develop the global value chain and trading market in time. And the timeline is important, as the last United Nation’s emission gap report [41] made very clear. Even based on the announced net zero ambitions of 50 states worldwide, the world is still on a path to a global temperature rise of between 2.5 and 3 °C.
As a global leading producer of specialty chemicals and catalysts Clariant is well positioned, and highly committed, to serve the evolving Power-to-X & Waste-to-X value chains, based on a broad material portfolio and corresponding know-how, which includes state-of-the-art catalysts.
Data availability
A multitude of informations is available on Clariant’s internet page: https://www.clariant.com/en/Business-Units/Catalysts.
References
Van der Hoeven M, Kobayashi Y, Diercks R (2013) Technology roadmap, energy and GHG reductions in the chemical industry via catalytic processes. IEA Dechema 56:16
IPCC, 2022: Climate Change 2022: Impacts, Adaptation and Vulnerability. Contribution of Working Group II to the Sixth Assessment Report of the Intergovernmental Panel on Climate Change [H.-O. Pörtner, D.C. Roberts, M. Tignor, E.S. Poloczanska, K. Mintenbeck, A. Alegría, M. Craig, S. Langsdorf, S. Löschke, V. Möller, A. Okem, B. Rama (eds.)]. Cambridge University Press. Cambridge University Press, Cambridge, UK and New York, NY, USA, 3056 pp. https://doi.org/10.1017/9781009325844
Cumulative CO2 emissions calculator - Engaging Data (engaging-data.com)
European Green Deal (europa.eu); https://climate.ec.europa.eu/eu-action/european-green-deal_en
EUR-Lex - 52022DC0230 - EN - EUR-Lex (europa.eu); https://eur-lex.europa.eu/legal-content/EN/TXT/?uri=COM%3A2022%3A230%3AFIN&qid=1653033742483
New EU framework to decarbonise gas markets (europa.eu) https://ec.europa.eu/commission/presscorner/detail/en/ip_21_6682; https://energy.ec.europa.eu/topics/energy-systems-integration/hydrogen_en
FH-National-Hydrogen-Strategies-Report-2022.pdf (fleishmanhillard.eu); https://fleishmanhillard.eu/wp-content/uploads/sites/7/2022/02/FH-National-Hydrogen-Strategies-Report-2022.pdf
Germany: transport sector energy consumption by source | Statista; https://www.statista.com/statistics/1312057/energy-consumption-transport-sector-germany-by-source/
Renewables (2020) Analysis and forecast to 2025 (windows.net); https://iea.blob.core.windows.net/assets/1a24f1fe-c971-4c25-964a-57d0f31eb97b/Renewables_2020-PDF.pdf
Hydrogen-Insights-2021.pdf (hydrogencouncil.com); https://hydrogencouncil.com/wp-content/uploads/2021/02/Hydrogen-Insights-2021.pdf
EUR-Lex - 52020DC0301 - EN - EUR-Lex (europa.eu); https://eur-lex.europa.eu/legal-content/EN/TXT/?uri=CELEX%3A52020DC0301
Hydrogen-Council-Report_Decarbonization-Pathways_Part-2_Supply-Scenarios.pdf (hydrogencouncil.com); https://hydrogencouncil.com/wp-content/uploads/2021/01/Hydrogen-Council-Report_Decarbonization-Pathways_Part-2_Supply-Scenarios.pdf
Preuster P, Papp C, Wasserscheid P (2017) Liquid organic hydrogen carriers (LOHCs): toward a hydrogen-free hydrogen economy. Acc Chem Res 50:74
Asinger F (2011) Methanol. Springer Verlag, Chemie- und Energierohstoff
Olah GA, Goeppert A, Surya Prakash GK (2006) Beyond oil and gas: the methanol economy. Wiley-VCH Verlag, Weinheim
Sterner M (2009) Bioenergy and renewable power methane in integrated 100% renewable energy systems. PhD thesis, Universität Kassel
Sabatier P, Senderens JB (1902) C R Acad Sci 134:689
Heat – Renewables 2019 – Analysis - IEA; https://www.iea.org/reports/renewables-2019/heat
Mapping the state of play of biomethane in Europe | European Biogas Association; https://www.europeanbiogas.eu/mapping-the-state-of-play-of-biomethane-in-europe/
Award-winning Partners in Clime (clariant.com); https://www.clariant.com/en/Corporate/Blog/2021-Blog-Posts/01/Amomax-Casale
Innovation Outlook: Renewable Methanol (irena.org); https://www.irena.org/publications/2021/Jan/Innovation-Outlook-Renewable-Methanol#:~:text=But%20with%20the%20right%20policies,methanol%20at%20a%20reasonable%20cost
Dechema Technology Study: Low carbon energy and feedstock for the European chemical industry, page 65
Innovation Outlook: Renewable Methanol (irena.org), page 62 table 11; https://www.irena.org/-/media/Files/IRENA/Agency/Publication/2021/Jan/IRENA_Innovation_Renewable_Methanol_2021.pdf?rev=ca7ec52e824041e8b20407ab2e6c7341; page 62 table 11
Irena and Methanol Institute (2021) Innovation outlook: renewable methanol. International Renewable Energy Agency, Abu Dhabi, p 12
Irena and Methanol Institute (2021) Innovation outlook: renewable methanol. International Renewable Energy Agency, Abu Dhabi, page 14
Van der Hoeven M, Kobayashi Y, Diercks R (2013) Technology roadmap, energy and GHG reductions in the chemical industry via catalytic processes. IEA Dechema 56:65
Bertau M, Offermanns H, Plass L, Schmidt F, Wernicke H-J (2014) Methanol: the basic chemical and energy feedstock of the future. Springer-Verlag, Berlin, Heidelberg
Nestler F, Full J, Jäckle J-M, Linsenmeier J, Roob J, Hadrich MJ, Schaadt A (2022) Experimental validation of methanol synthesis from steel mill gases using a miniplant setup. Chem Ing Tech 94:1466
Nestler F, Müller VP, Ouda M, Hadrich MJ, Schaadt A, Bajohr S, Kolb T (2021) A novel approach for kinetic measurements in exothermic fixed bed reactors: advancements in non-isothermal bed conditions demonstrated for methanol synthesis. React Chem Eng 6:1092
Irena and Methanol Institute (2021) Innovation Outlook: renewable methanol. International Renewable Energy Agency, Abu Dhabi, p 85 (Figure 41)
Irena and Methanol Institute (2021) Innovation Outlook: Renewable Methanol. International Renewable Energy Agency, Abu Dhabi, p 86
Irena and Methanol Institute (2021) Innovation outlook: renewable methanol. International Renewable Energy Agency, Abu Dhabi, p 57
HIF Chile (hifglobal.com); https://www.hifglobal.com/hif-chile
Fit for 55 - The EU's plan for a green transition - Consilium (europa.eu); https://www.consilium.europa.eu/en/policies/green-deal/fit-for-55-the-eu-plan-for-a-green-transition/
ReFuelEU Aviation initiative: Sustainable aviation fuels and the fit for 55 package | Think Tank | European Parliament (europa.eu); https://www.europarl.europa.eu/thinktank/en/document/EPRS_BRI(2022)698900
ExxonMobil methanol to jet technology to provide new route for sustainable aviation fuel production (exxonmobilchemical.com); BMDV-funded M2SAF development project for the production of sustainable aviation fuel from methanol successfully launched (thyssenkrupp-uhde.com)
INERATEC and Clariant: Joined forces for a cleaner future | INERATEC; https://www.ineratec.de/en/en/ineratec-and-clariant-joined-forces-for-a-cleaner-future; power-to-liquid pioneer plant - Infraserv; https://www.infraserv.com/en/company/sustainability/power-to-liquid-pioneer-plant/
Data source: World Energy Outlook 2018, IEA, New Policies & Sustainable Development Scenario
https://www.ipcc.ch/report/ar6/wg1/downloads/report/IPCC_AR6_WGI_SPM_final.pdf
Emissions Gap Report 2021 (unep.org); https://www.unep.org/resources/emissions-gap-report-2021
Author information
Authors and Affiliations
Corresponding author
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Berchthold, C., Geisbauer, A. Will Power and Waste-to-X enable the worldwide energy transition to renewables?. Monatsh Chem (2024). https://doi.org/10.1007/s00706-024-03176-6
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s00706-024-03176-6