Abstract
Due to the increasing demand for lithium-ion batteries, there is an urgent requirement for environmentally friendly and efficient means of recycling these batteries. Graphite, a readily available and cost-effective material, tends to be neglected compared to more expensive metals such as cobalt or nickel. To achieve the new European targets, it will be necessary to focus on recycling even less valuable materials, such as graphite. Direct recycling of graphite represents an environmentally and economically viable solution. However, the capacity of recycled graphite depends on several factors, with pressing pressure being a potential variable. Within this article, we have focused on the impact of pressing pressure of spent graphite anode. The recycling was performed on the battery sample with a known lifetime history. It was found that when optimized, it is possible to achieve high stability and high capacities exceeding 300 mAh/g.
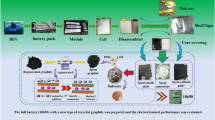
Graphical abstract

Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Approximately 11 million tons of discarded lithium-ion batteries (LIBs) are expected to accumulate by 2030 and the annual EV battery waste stream is projected to reach 34,000 tons by 2040. Sustainable and efficient recycling of these batteries is essential. Traditional recycling methods leads to significant greenhouse gas emissions, and energy consumption. In contrast, emerging direct recycling processes offer promising alternatives as they rejuvenate LIB compounds without changing their chemical composition or embodied energy [1].
The predominant cathode technologies are LiNi1-x-yMnxCoyO2 (NMC) and LiNi1-x-yCoxAlyO2 (NCA). These cathodes have a significant market share, with cobalt-free alternatives such as LiFePO4 (LPF) becoming increasingly popular [2, 3].
Graphite as anode material holds a significant position in the commercial LIBs market due to its exceptional electrochemical properties and wide availability [4, 5]. Graphite as an anode material is used in 97% of all batteries produced [6]. However, graphite gradually degrades during cycling. The repetitive process of intercalation and deintercalation of lithium ions in the graphite anode leads to volumetric fluctuations (up to 13.2%) [7] that eventually lead to stress-induced cumulative cracking and fragmentation of graphite particles [6, 8].
The pyrometallurgical process, unlike direct recycling, does not recycle graphite at all. Instead, graphite is often used to reach the melting temperature in a high furnace [9, 10]. The hydrometallurgical recycling process can be used to obtain pure graphite, but this process is often used after the pyrometallurgical process to recover metals from the slag [11, 12]. When attempting to recycle graphite directly, emphasis is often placed on cleaning the material and restoring its crystalline structure [1, 13].
The initial stage of renovating used graphite involves removing impurities that include solid electrolyte interphase (SEI) residues, which is formed from electrolyte [14], polymer binders, conductive materials, contaminants, and metallic residues. Various techniques are used to remove impurities, including heat treatment, chemical treatment, acid treatment, and water treatment [1, 15].
Anode material from a discarded battery also contains significant amounts of lithium. Therefore, recycling the anode can provide a valuable source not only of graphite but also of lithium. For this reason, it is believed that lithium recycling is indispensable [15].
There are several papers on graphite recycling, but none of them address the effect of press pressure on capacity, for example, Tian et al. [16]. It uses a synergistic combination of thermal and hydrometallurgical operations to remove binder and impurities from graphite anodes. The author uses multiple samples for recycling and declares an increase in capacity. However, he does not mention the pressing pressures of the electrodes he used for electrochemical experiments.
Wang et al. [17] regenerated graphite by simple water treatment. The resulting electrode contains 80% graphite, 10% PVDF, and 10% conductive additives. The author presents a capacity of 345 mAh/g.
Lithium iron phosphate (LFP) batteries have made significant inroads in both electric vehicles and stationary energy storage applications, where they have been used for more than 10 years. As LFP batteries continue to be adopted at a rapid pace [18], the need for efficient recycling and disposal methods is becoming increasingly critical. In the near future, investing in advanced recycling technologies and establishing robust recycling infrastructures will be crucial to address the growing volume of end-of-life LFP batteries. In this paper, we have focused on direct recycling of graphite from spent LFP batteries and the effect of pressing pressure on the capacity of recycled graphite. The issue of the effect of pressing pressure in the fabrication of electrodes based on recycled graphite has not yet been described in the literature, and in this paper, we found that this parameter has a significant effect on the electrochemical performance in terms of capacity and stability during long-term cycling or at higher loads.
Results and discussion
The initial capacity at 1 C-rate cycling of the Motoma LFP battery was 1485 mAh; see Fig. 1A. After 500 cycles, the capacity decreased to 1375 mAh. The decrease in capacity between the first and last cycle was 7.5%. The capacity of the Motoma LFP at 1 °C cycling was constant for 175 cycles. From 175 cycles onwards, the capacity started to decrease in a linear trend. Coulombic efficiency was around 100% throughout the cycling period.
The discharge characteristic curve of a Motoma LFP cell, see Fig. 1B, is typical curve for LFP cathode material. The initial voltage drop is followed by a stable plateau around 3.3 V. The discharge capacity at 0.1 C was 1590 mAh. The capacity at 0.1 °C charge/discharge rate of cycled battery at 1 C for 500 cycles dropped by 9.4% to 1450 mAh.
After cell disassembly (cell was previously discharged to 1 V), anode extraction was graphite separated from the current collector by demineralized water and drayed. Extracted graphite was subsequently analyzed by SEM and EDS analysis. Figure 1A shows SEM image of the extracted graphite particles. It is evident that their size ranges up to 15 µm. A detail of a 5 µm graphite particle with a typical graphite shape is shown in Fig. 2B.
As can be seen in Fig. 3, from EDS analysis, it is evident that this is essentially pure graphite with no electrolyte residue. Traces of oxygen are observed on the surface of the graphite particles, which will be adsorbed atmospheric oxygen that was adsorbed during the handling of the graphite. However, Fig. 3D shows calcium particles that were probably already present from the manufacture of the battery as an impurity during the manufacturing process. From the EDS analysis of the extracted graphite sample was observed that 95.46% of the mass composition is made by carbon, 4.32% by oxygen, and 0.22% calcium.
The recycled graphite electrodes were then produced from the extracted graphite and pressed under different pressures (1843 N/cm2, 3840 N/cm2, 5684 N/cm2, and 7681 N/cm2). Since it is practically impossible to find the pressing pressure for anodes in the literature, the pressing pressure was selected in the range of low to high based on previous experience within the laboratory to avoid delamination of the active layer. Cyclic voltammetry was measured in ElCell set up with a scan rate of 0.1 mV/s. From the CV curves shown in Fig. 4, it is clearly evident that with increasing pressure, the current of the anodic peak decreases, and at the same time, it shifts to a higher potential. At a pressure of 1843 N/cm2, the maximum anodic current was 3.6 mA/g at a voltage of 0.25 V. In contrast, at a pressure of 7681 N/cm2, the maximum value of the current was 3.2 mA/g at voltage 0.27 V. As the pressing pressure increases, the peak height decreases, the peak broadens and the stability decreases. CV was followed by long-term cycling at different loads from 0.1 to 1 °C. We can see this cycling for all pressing pressures in Fig. 5.
As shown in Fig. 5A, recycled graphite electrode pressed by 1843 N/cm2 achieved a capacity of 318 mAh/g in the first cycle of cycling at 0.1 C, which is a higher capacity than the capacity achieved by the graphite anode after cycling the 18,650 cell (257 mAh/g). After the first ten cycles with a current of 0.1 °C, the electrode pressed with a pressure of 1843 N/cm2 shows decrease of capacity by 2.5% to 310 mAh/g. When the load was increased to 0.2 °C, the capacity decreased to 287 mAh/g. At 0.5 C load, the capacity dropped to 242 mAh/g, and at the highest load 1 °C decreased to 136 mAh/g, what corresponds to a decrease of 58%. Subsequently, when the load was reduced, the capacity returned to its original values and was very stable. When the load was reduced to 0.1 °C, the capacity returned to 292 mAh/g, while the capacity drop after 40 cycles was 8.2%.
Figure 5B shows recycled graphite electrode pressed by 3840 N/cm2. For this, pressing pressure was achieved capacity of 300 mAh/g in the first cycle of cycling at 0.1 °C. After the first ten cycles with a current of 0.1 °C, the electrode shows decrease of capacity by 9.0% to 273 mAh/g. When the load was increased to 0.2 °C, the capacity decreased to 237 mAh/g. At 0.5 °C load, the capacity dropped to 148 mAh/g, and at the highest load 1 °C decreased to 47 mAh/g, what corresponds to a decrease of 84.5%. Subsequently, when the load was reduced, the capacity was increasing with decreasing C-rate, but capacity did not reach the original values. When the load was reduced to 0.1 °C, the capacity stabilized at 236 mAh/g, while the capacity drop after 40 cycles was 24.4%.
Figure 5C shows recycled graphite electrode pressed by 5684 N/cm2. For this, pressing pressure was achieved capacity of 262 mAh/g in the first cycle of cycling at 0.1 °C. After the first 10 cycles with a current of 0.1 °C, the electrode shows rapid decrease of capacity by 30.2% to 183 mAh/g. When the load was increased to 0.2 °C, the capacity decreased to 95 mAh/g. At 0.5 °C load, the capacity fell to 16 mAh/g, what corresponds to a decrease of 94%. In terms of capacity drop, the experiment was terminated after 0.5 °C.
Figure 5D shows recycled graphite electrode pressed by 7681 N/cm2. For this, pressing pressure was achieved capacity of 289 mAh/g in the first cycle of cycling at 0.1 °C. After the first ten cycles with a current of 0.1 °C, the electrode shows gradual decrease of capacity by 14.5% to 247 mAh/g. When the load was increased to 0.2 °C, the capacity decreased to 171 mAh/g. At 0.5 °C load, the capacity massively fell to 32 mAh/g. 1 °C load cycling was performed with final capacity of 8 mAh/g, what corresponds to a decrease of 97.5%. Due to capacity drop, the experiment was subsequently terminated.
Figure 6 shows the charge/discharge characteristics of recycled graphite electrodes created using different pressing pressures at 0.1 °C. The data obtained from the charge/discharge characteristics are in line with the data obtained by CV, where the highest capacity reached correlates with the highest current peaks of CV. Thus, the highest capacity (318 mAh/g) is achieved by the electrode with lowest pressing pressure of 1843 N/cm2.
It was also found that the pressing pressure affects the hysteresis of the electrodes. The hysteresis between charge/discharge curve at 50% of its capacity was 0.13 V for a pressing pressure of 1843 N/cm2. With increasing pressing pressure, the hysteresis increased to 0.14 V, 0.28 V, and 0.19 V for pressing pressures of 3840 N/cm2, 5684 N/cm2, and 7681 N/cm2, respectively. This behavior is similar to the decrease of achieved capacity with pressing pressure increase. This trend corelates with CV, where the position of the anodic peak increases with increasing pressing pressure, similarly as the hysteresis increases. As the pressing pressure increases, the current decreases, similar to the decrease in capacity during cycling.
Conclusion
Recycling of spent graphite anode by direct recycling method appears to be a viable approach. Water used as a solvent for the anode electrode is cost-effective and widely available. From the cyclic voltammetry results, the stability of the newly fabricated recycled graphite electrodes can be observed, with lower pressing pressures showing greater stability. From the discharge characteristics, it can be observed the high achieved capacity of the electrodes, which is over 300 mAh/g and therefore capacity was increased by 12% compared to the capacity of the graphite in the original battery. Cycling at different loads shows highest capacity and minimal capacity loss for a pressing pressure of 1843 N/cm2. From the results of this work, it can be concluded that an anode made of recycled graphite with suitable pressing pressure can be used for the production of new more sustainable batteries. Moreover, by recycling the graphite, the overall efficiency of the recycling process could be significantly increased, as graphite accounted for 15.4% of the total weight of the used LFP Li-ion battery.
Experimental
The battery utilized in this study was a commercially available cylindrical cell Motoma LFP 18650 (1550 mAh), featuring an LFP cathode and graphite anode.
Cell characteristics
The battery was cycled using the CCCV method to determine the initial properties. Charging/discharging was performed at 0.1 °C in voltage range from 2.5 to 4.2 V.
To replicate the battery's aging process, an extended cycling test was performed. Charge/discharge measurements were performed using the constant-current-constant-voltage (CCCV) mode. Cycling was performed in 1 C-rate in voltage range from 2.5 to 4.2 V, totaling 500 cycles. After 500 cycles, battery was again subjected to galvanostatic cycling at 0.1 °C.
Cell recycling
Subsequently, the battery was discharged to 1 V with 0.1 °C current. Afterward, the outer metal casing was removed and the individual layers were separated. After evaporation of the electrolyte, mass of anode, the surface, and the thickness of anode, i.e., the thickness of the current collector was measured. Graphite content was calculated to be 6.156 g out of a total battery weight of 40.1 g. Hence, the approximate capacity of graphite in the Motoma LFP battery after aging was calculated to be 234 mAh/g. If we consider that for anode material in commercial Li-ion battery is typical excess amount of graphite (about 10%), the capacity would be 257.4 mAh/g. Second consideration would be that the anode is 90% graphite and the remaining 10% is binder and conductive additives, the total capacity increases to 283.14 mAh/g. Subsequently, the copper anode with graphite layer was separated using water as solvent. The suspension was filtered and dried in an oven at 60 °C for 24 h. The dried graphite was hand ground using an agate friction pan.
The dried graphite anode was subjected to SEM and EDS analysis. The analysis shows that the anode is free of electrolyte and binder residues and is virtually pure graphite at 95.46%. 4.32% is oxygen and 0.22% of the anode is calcium. Calcium content is probably pollution from the manufacturing process. Electron microscope used was TESCAN VEGA3 XMU and a Bruker EDAX analyzer.
Electrochemical testing
Water-washed and dried graphite was used for the production of new electrodes. The slurry was composed of 90% graphite and 10% polyvinylidene fluoride (PVDF, Sigma-Aldrich) binder. The slurry was prepared using a magnetic stirrer to mix 900 mm3 of N-methylpyrrolidone (NMP, Sigma-Aldrich) solvent with PVDF binder. After dissolution of PVDF, graphite was added and mixed for 24 h. For this slurry, none conductive carbon powder or other additives were added. After 24 h of mixing, slurry was deposited with coating bar with 200 µm thickness onto a thin copper current collector. The average graphite load is around 4.27 mg/cm2. Deposited copper collector with graphite was then dried for 24 h in oven at 60 °C. After drying, the electrodes were cut out using an 18 mm-diameter cutter. These electrodes were then exposed to four different pressing pressures: 1843 N/cm2, 3840 N/cm2, 5684 N/cm2, and 7681 N/cm2. After pressing, electrodes were dried in vacuum oven for 24 h at 110 °C to remove possible moisture. After drying, the electrodes were moved to the Jacomex glowebox with Ar-filled atmosphere, where electrochemical cells were assembled. Lithium metal counter electrode was placed in the El-cell, covered with a glass separator, and filled by 1 M LiPF6 salt dissolved in DMC (dimethyl carbonate) and EC (ethylene carbonate) (1:1, v:v). Recycled graphite electrodes were used as working electrode.
CV was performed with a scan rate of 0.1 mV/s in the potential range from 0.01 to 2.5 V vs. Li/Li+. Galvanostatic cycling was performed in the voltage range from 0.01 to 2.5 V vs. Li/Li+ at different C-rates from 0.1 to 1 °C. All electrochemical test was performed on Biologic VMP3 with Booster and a ZKETECH EBC-X cycler.
Data availability
The data that support the findings of this study are available from the corresponding author, Jiří Báňa, upon reasonable request.
References
Wu J, Zheng M, Liu T, Wang Y, Liu Y, Nai J, Zhang L, Zhang S, Tao X (2023) Energy Storage Mater 54:120
Loghavi M, Nahvibayani A, Moghim M, Babaiee M, Baktashian S, Eqra R (2022) Monatsh Chem 153:1197
Salgado R, Danzi F, Oliveira J, El-Azab A, Camanho P, Braga M (2021) Molecules 26:3188
Natarajan S, Aravindan V (2020) Adv Energy Mater 10:2002238
Pagliaro M, Meneguzzo F (2019) Heliyon 5:e01866
Mills R (2022) Graphite deficit starting this year, as demand for EV battery anode ingredient exceeds supply. https://www.mining.com/web/graphite-deficit-starting-this-year-as-demand-for-ev-battery-anode-ingredient-exceeds-supply/
Schweidler S, de Biasi L, Schiele A, Hartmann P, Brezesinski T, Janek J (2018) J Phys Chem C 122:8829
Edge J, O’Kane S, Prosser R, Kirkaldy N, Patel A, Hales A, Ghosh A, Ai W, Chen J, Yang J, Li S, Pang M, Bravo Diaz L, Tomaszewska A, Marzook M, Radhakrishnan K, Wang H, Patel Y, Wu B, Offer G (2021) Phys Chem Chem Phys 23:8200
Baum Z, Bird R, Yu X, Ma J (2022) ACS Energy Lett 7:712
Qu G, Yang J, Wang H, Ran Y, Li B, Wei Y (2023) Waste Manage 166:222
Larouche F, Tedjar F, Amouzegar K, Houlachi G, Bouchard P, Demopoulos G, Zaghib K (2020) Materials 13:801
Guimarães L, Botelho Junior A, Espinosa D (2022) Miner Eng 183:107597
Ji Y, Kpodzro E, Jafvert C, Zhao F (2021) Clean Technol Recycl 1:124
Winter M, Appel W, Evers B, Hodal T, Möller K, Schneider I, Wachtler M, Wagner M, Wrodnigg G, Besenhard J (2001) Monatsh Chem 132:473
Lu Y, Peng K, Zhang L (2022) ACS ES&T Eng 2:586
Tian H, Graczyk-Zajac M, De Carolis D, Tian C, Ricohermoso E, Yang Z, Li W, Wilamowska-Zawlocka M, Hofmann J, Weidenkaff A, Riedel R (2023) J Hazardous Mater 445:130607
Wang H, Huang Y, Huang C, Wang X, Wang K, Chen H, Liu S, Wu Y, Xu K, Li W (2019) Electrochim Acta 313:423
Xu P, Dai Q, Gao H, Liu H, Zhang M, Li M, Chen Y, An K, Meng Y, Liu P, Li Y, Spangenberger J, Gaines L, Lu J, Chen Z (2020) Joule 4:2609
Acknowledgements
This work was supported by the specific graduate research of the Brno University of Technology under Grant No. FEKT-S-23-8286.
Funding
Open access publishing supported by the National Technical Library in Prague.
Author information
Authors and Affiliations
Corresponding author
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Báňa, J., Čudek, P., Šedina, M. et al. Effect of pressing pressure on the capacity of recycled graphite anode. Monatsh Chem 155, 253–259 (2024). https://doi.org/10.1007/s00706-024-03174-8
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00706-024-03174-8